CBSE Sample Paper-02
SUMMATIVE ASSESSMENT –I
SCIENCE (Theory)
Class – X
Time allowed: 3 hours Maximum Marks: 90
General Instructions:
a) All questions are compulsory.
b) The question paper comprises of two sections, A and B. You are to attempt both the sections.
c) Questions 1 to 3 in section A are one mark questions. These are to be answered in one word
or in one sentence.
d) Questions 4 to 6 in section A are two marks questions. These are to be answered in about 30
words each.
e) Questions 7 to 18 in section A are three marks questions. These are to be answered in about
50 words each.
f) Questions 19 to 24 in section A are five marks questions. These are to be answered in about
70 words each.
g) Questions 25 to 27 in section B are 2 marks questions and Questions 28 to 36 are multiple
choice questions based on practical skills. Each question of multiple choice questions is a one
mark question. You are to select one most appropriate response out of the four provided to
you.
1.Why does milk become sour if kept for a long time?
2. Name the respiratory organs of: (i) fish, (ii) mosquito, (iii) earthworm.
3. Name a metal which offers higher resistance to the passage of electricity other than copper.
4. (i) An aqueous solution has a pH value of 7.0. Is this solution acidic, basic or neutral?
(ii) Which has a higher pH value, 1 M HCl or 1 M NaOH solution?
5. Taking the example of auxins and cytokinins together, explain (i) a synergistic action in plants,
(ii) an antagonistic action in plants.
6. A wire carrying current is passing through a hole at the middle of a cardboard. Plot the
magnetic field lines.
7. What information can be included in a chemical reaction?
8. What happens when Zn metal is dipped in CuSO4 solution? Give the chemical reaction involved.
State which is more reactive, Zn or Cu?
9. (a) Name the raw material used in the manufacture of sodium carbonate by Solvay process.
(b) How is the sodium hydrogen carbonate formed during Solvay process separated from a
mixture of NH4Cl and NaHCO3?
(c) How is sodium carbonate obtained from sodium hydrogen carbonate?
10. (a) Explain the term ‘roasting’ as used in metallurgical processes. Give one suitable example for
it.
(b) What changes takes place when Cinnabar (HgS) is heated in air for a long enough time?
11.Reasons for the following:
(a) Metals are good conductors of heat.
(b) Addition of some silver to pure gold for making ornaments.
(c) Inability of non-metals for displacing hydrogen from dilute sulphuric acid.
12. Name the three kinds of cells present in blood. Write one function each of them.
13. Draw a diagram of human alimentary canal showing duodenum, small intestine, liver and
pancreas.
14. Draw a diagram of human brain and label the following parts:
(a) Cerebrum (b) Meninges (c) Medulla oblongata (d) Cerebellum
15. Vikalp’s father had constructed a new room in their house. An electrician was called in to do
the electric wiring. The electrician was asked to do wiring for two fans, two bulbs, a light
socket and a power socket. Vikalp studies in tenth standard. Just when the electrician had
completed the wiring, Vikalp returned home from school. Vikalp wanted to check the wiring by
using all the switches and sockets. Vikalp found that the two fans and two sockets worked
properly, each having a separate switch but there was a problem in the working of bulbs. Both
the bulbs could be switched on and switched off with the same switch. Vikalp explained the
mistake in wiring to electrician and then two separate switches were provided for the two
bulbs.
Read the above passage and answer the following questions:
(a) In what way were the two fans and two sockets connected in the household circuit by
electrician?
(b)What mistake made by the electrician in connecting two bulbs in the circuit?
(c) What values were displayed by Vikalp during this incident?
[Value Based Question]
16. Draw the pattern of field lines due to a bar magnet. Mention any two properties of the
magnetic field lines.
17. (a) Name the device used to convert:
(i) Solar energy into heat and
(ii) Solar energy into electricity.
(b) Explain the principle of working of a wind mill.
18. Name three forms in which energy from ocean is made available for use. What are OTEC power
plants? How do they operate?
19. Write the balanced chemical equations for the following reactions:
(i) Calcium hydroxide + Carbon dioxide ¾¾® Calcium carbonate + Water
(ii) Zinc + Silver nitrate ¾¾® Zinc nitrate + Silver
(iii) Aluminium + Chromium oxide ¾¾® Aluminium oxide + Chromium
(iv) Barium chloride + Potassium sulphate ¾¾® Barium sulphate + Potassium chloride
(v) Hydrogen + Chlorine ¾¾® Hydrogen chloride
Or
Give the characteristic tests for the following gases:
(i) CO2 (ii) SO2 (iii) O2 (iv) H2
20.Differentiate between an alloy and an amalgam. How are alloys made? State with examples any
two properties in which an alloy may be different from those of its constituents. Write the
constituents and special advantages of:
(i) Stainless Steel (ii) Magnalium
Or
What is meant by the term “Enrichment of Ore”? Name four methods generally used for
enrichment of ore. With the help of a labelled diagram, describe the method for the enrichment
of sulphide ore.
21. Define the terms ‘Nutrition’ and ‘Nutrients’. List two differences between ‘Holozoic nutrition’
and ‘Saprophytic nutrition’. Give two examples of each of these two types of nutrition.
Or
(a) Explain why the rate of photosynthesis in plant is low both of lower and higher
temperature.
(b) Is green light most or least useful in photosynthesis and why?
(c) Describe an activity to show that chlorophyll is necessary for photosynthesis in plants.
22. Draw the schematic diagram of a circuit containing the following electrical equipments:
(i) a resistance (ii) a voltmeter (iii) an electric bulb
(iv) a cell (v) plug key (open) (vi) an ammeter
Or
Three incandescent bulbs of 100 W each are connected in series in an electric circuit. In other
set of three bulbs of the same wattage are connected in parallel to the source.
(a) Will the bulb in the two circuits glow with the same brightness? Justify your answer.
(b) Now, let one bulb in both the circuits get fused. Will the rest of the bulbs continue to glow
in each circuit? Give reason.
23. State ‘Fleming’s Right hand rule’. With a labelled diagram, describe the working of an A.C.
electric generator.
Or
Explain with neat and labelled diagram, the principle, construction and working of D.C.
generator, showing the output.
24. Draw the line of forces (indicating field direction) of the magnetic field through and around (a)
a single loop wire carrying electric current and (b) a solenoid carrying electric current.
Or
(a) Draw a schematic diagram of a domestic electric circuit which includes a main fuse, a
power meter, a light point, a fan and a power plug.
(b)Why is it necessary to earth the metallic electric appliances?
Section B
25. When red litmus paper is added to limewater, then what will be the change in litmus paper?
Give reason. Write the chemical formula of limewater also.
26. A destarched leaf on a potted plant was covered with black (A), white (B) and transparent (C)
strips of paper as shown in the figure.
After six hours to exposure to sunlight the leaf was removed from the plant and tested for
starch.
(a) What changes will be observed?
(b) Justify your answer.
27. A student draw the following circuit diagram for the experiment on studying the dependence
of current (I) on potential difference (V) across a resistor.
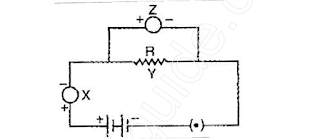
What are the parts labelled X, Y and
Z in this diagram respectively? Justify your answer also.
28. When the colour of pH paper becomes red, the solution is _________ and pH is between _________.
(a) strongly acid, pH = 1 to 2 (b) weakly acid, pH = 6 to 7
(c) strongly basic, pH = 12 to 13 (d) weakly basic, pH = 7 to 8
29. When SO2 gas is passed through acidified K2Cr2O7 solution:
(a) The solution becomes green due to formation of K2SO4.
(b) The solution becomes green due to formation of Cr2(SO4)2.
(c) The solution becomes yellow due to formation of K2SO4.
(d) The solution becomes red due to formation of Cr2(SO4)2.
30. SO2 gas should not be inhaled because:
(a) It is poisonous.
(b) It is acidic in nature.
(c) It is lighter than air.
(d) It is pungent smelling.
31. Growth hormone : Pituitary : Thyroxin : __________
(a) Thyroid (b) Parathyroid (c) Pancreas (d) Adernal
32. How many spinal nerves are present in human being:
(a) 31 pairs (b) 19 pairs (c) 27 pairs (d) 30 pairs
33. The rest positions of the needles in a Milliammeter and Voltmeter when not being used in a
circuit are as shown in the figure. The ‘zero error’ and ‘least count’ of these two instruments
are:
(a) (+4 mA, –0.2 V) and (1 mA, 0.1 V) respectively
(b) (+4 mA, –0.2 V) and (2 mA, 0.2 V) respectively
(c) (–4 mA, +0.2 V) and (2 mA, 0.2 V) respectively
(d) (–4 mA, +0.2 V) and (2 mA, 0.1 V) respectively
34. While performing the experiment on studying the dependence of current (I) on the potential
difference (V) across a resistor, four students I, II, III and IV set up the circuit is shown.
The correct result will be obtained by the student.
(a) I (b) II (c) III (d) IV
35. If the key in the arrangement is taken out (the circuit is made open) and magnetic field lines
are drawn over the horizontal plane, the lines are:
(a) concentric circles.
(b) elliptical in shape.
(c) straight lines parallel to each other.
(d) concentric circles near the point O but of elliptical shapes as we go away from it.
36. Fuel used in thermal power plants is:
(a) water (b) uranium (c) biomass (d) fossil fuels
SUMMATIVE ASSESSMENT –I
SCIENCE (Theory)
Class – X
Time allowed: 3 hours Maximum Marks: 90
General Instructions:
a) All questions are compulsory.
b) The question paper comprises of two sections, A and B. You are to attempt both the sections.
c) Questions 1 to 3 in section A are one mark questions. These are to be answered in one word
or in one sentence.
d) Questions 4 to 6 in section A are two marks questions. These are to be answered in about 30
words each.
e) Questions 7 to 18 in section A are three marks questions. These are to be answered in about
50 words each.
f) Questions 19 to 24 in section A are five marks questions. These are to be answered in about
70 words each.
g) Questions 25 to 27 in section B are 2 marks questions and Questions 28 to 36 are multiple
choice questions based on practical skills. Each question of multiple choice questions is a one
mark question. You are to select one most appropriate response out of the four provided to
you.
1.Why does milk become sour if kept for a long time?
2. Name the respiratory organs of: (i) fish, (ii) mosquito, (iii) earthworm.
3. Name a metal which offers higher resistance to the passage of electricity other than copper.
4. (i) An aqueous solution has a pH value of 7.0. Is this solution acidic, basic or neutral?
(ii) Which has a higher pH value, 1 M HCl or 1 M NaOH solution?
5. Taking the example of auxins and cytokinins together, explain (i) a synergistic action in plants,
(ii) an antagonistic action in plants.
6. A wire carrying current is passing through a hole at the middle of a cardboard. Plot the
magnetic field lines.
7. What information can be included in a chemical reaction?
8. What happens when Zn metal is dipped in CuSO4 solution? Give the chemical reaction involved.
State which is more reactive, Zn or Cu?
9. (a) Name the raw material used in the manufacture of sodium carbonate by Solvay process.
(b) How is the sodium hydrogen carbonate formed during Solvay process separated from a
mixture of NH4Cl and NaHCO3?
(c) How is sodium carbonate obtained from sodium hydrogen carbonate?
10. (a) Explain the term ‘roasting’ as used in metallurgical processes. Give one suitable example for
it.
(b) What changes takes place when Cinnabar (HgS) is heated in air for a long enough time?
11.Reasons for the following:
(a) Metals are good conductors of heat.
(b) Addition of some silver to pure gold for making ornaments.
(c) Inability of non-metals for displacing hydrogen from dilute sulphuric acid.
12. Name the three kinds of cells present in blood. Write one function each of them.
13. Draw a diagram of human alimentary canal showing duodenum, small intestine, liver and
pancreas.
14. Draw a diagram of human brain and label the following parts:
(a) Cerebrum (b) Meninges (c) Medulla oblongata (d) Cerebellum
15. Vikalp’s father had constructed a new room in their house. An electrician was called in to do
the electric wiring. The electrician was asked to do wiring for two fans, two bulbs, a light
socket and a power socket. Vikalp studies in tenth standard. Just when the electrician had
completed the wiring, Vikalp returned home from school. Vikalp wanted to check the wiring by
using all the switches and sockets. Vikalp found that the two fans and two sockets worked
properly, each having a separate switch but there was a problem in the working of bulbs. Both
the bulbs could be switched on and switched off with the same switch. Vikalp explained the
mistake in wiring to electrician and then two separate switches were provided for the two
bulbs.
Read the above passage and answer the following questions:
(a) In what way were the two fans and two sockets connected in the household circuit by
electrician?
(b)What mistake made by the electrician in connecting two bulbs in the circuit?
(c) What values were displayed by Vikalp during this incident?
[Value Based Question]
16. Draw the pattern of field lines due to a bar magnet. Mention any two properties of the
magnetic field lines.
17. (a) Name the device used to convert:
(i) Solar energy into heat and
(ii) Solar energy into electricity.
(b) Explain the principle of working of a wind mill.
18. Name three forms in which energy from ocean is made available for use. What are OTEC power
plants? How do they operate?
19. Write the balanced chemical equations for the following reactions:
(i) Calcium hydroxide + Carbon dioxide ¾¾® Calcium carbonate + Water
(ii) Zinc + Silver nitrate ¾¾® Zinc nitrate + Silver
(iii) Aluminium + Chromium oxide ¾¾® Aluminium oxide + Chromium
(iv) Barium chloride + Potassium sulphate ¾¾® Barium sulphate + Potassium chloride
(v) Hydrogen + Chlorine ¾¾® Hydrogen chloride
Or
Give the characteristic tests for the following gases:
(i) CO2 (ii) SO2 (iii) O2 (iv) H2
20.Differentiate between an alloy and an amalgam. How are alloys made? State with examples any
two properties in which an alloy may be different from those of its constituents. Write the
constituents and special advantages of:
(i) Stainless Steel (ii) Magnalium
Or
What is meant by the term “Enrichment of Ore”? Name four methods generally used for
enrichment of ore. With the help of a labelled diagram, describe the method for the enrichment
of sulphide ore.
21. Define the terms ‘Nutrition’ and ‘Nutrients’. List two differences between ‘Holozoic nutrition’
and ‘Saprophytic nutrition’. Give two examples of each of these two types of nutrition.
Or
(a) Explain why the rate of photosynthesis in plant is low both of lower and higher
temperature.
(b) Is green light most or least useful in photosynthesis and why?
(c) Describe an activity to show that chlorophyll is necessary for photosynthesis in plants.
22. Draw the schematic diagram of a circuit containing the following electrical equipments:
(i) a resistance (ii) a voltmeter (iii) an electric bulb
(iv) a cell (v) plug key (open) (vi) an ammeter
Or
Three incandescent bulbs of 100 W each are connected in series in an electric circuit. In other
set of three bulbs of the same wattage are connected in parallel to the source.
(a) Will the bulb in the two circuits glow with the same brightness? Justify your answer.
(b) Now, let one bulb in both the circuits get fused. Will the rest of the bulbs continue to glow
in each circuit? Give reason.
23. State ‘Fleming’s Right hand rule’. With a labelled diagram, describe the working of an A.C.
electric generator.
Or
Explain with neat and labelled diagram, the principle, construction and working of D.C.
generator, showing the output.
24. Draw the line of forces (indicating field direction) of the magnetic field through and around (a)
a single loop wire carrying electric current and (b) a solenoid carrying electric current.
Or
(a) Draw a schematic diagram of a domestic electric circuit which includes a main fuse, a
power meter, a light point, a fan and a power plug.
(b)Why is it necessary to earth the metallic electric appliances?
Section B
25. When red litmus paper is added to limewater, then what will be the change in litmus paper?
Give reason. Write the chemical formula of limewater also.
26. A destarched leaf on a potted plant was covered with black (A), white (B) and transparent (C)
strips of paper as shown in the figure.
After six hours to exposure to sunlight the leaf was removed from the plant and tested for
starch.
(a) What changes will be observed?
(b) Justify your answer.
27. A student draw the following circuit diagram for the experiment on studying the dependence
of current (I) on potential difference (V) across a resistor.
What are the parts labelled X, Y and
Z in this diagram respectively? Justify your answer also.
28. When the colour of pH paper becomes red, the solution is _________ and pH is between _________.
(a) strongly acid, pH = 1 to 2 (b) weakly acid, pH = 6 to 7
(c) strongly basic, pH = 12 to 13 (d) weakly basic, pH = 7 to 8
29. When SO2 gas is passed through acidified K2Cr2O7 solution:
(a) The solution becomes green due to formation of K2SO4.
(b) The solution becomes green due to formation of Cr2(SO4)2.
(c) The solution becomes yellow due to formation of K2SO4.
(d) The solution becomes red due to formation of Cr2(SO4)2.
30. SO2 gas should not be inhaled because:
(a) It is poisonous.
(b) It is acidic in nature.
(c) It is lighter than air.
(d) It is pungent smelling.
31. Growth hormone : Pituitary : Thyroxin : __________
(a) Thyroid (b) Parathyroid (c) Pancreas (d) Adernal
32. How many spinal nerves are present in human being:
(a) 31 pairs (b) 19 pairs (c) 27 pairs (d) 30 pairs
33. The rest positions of the needles in a Milliammeter and Voltmeter when not being used in a
circuit are as shown in the figure. The ‘zero error’ and ‘least count’ of these two instruments
are:
(a) (+4 mA, –0.2 V) and (1 mA, 0.1 V) respectively
(b) (+4 mA, –0.2 V) and (2 mA, 0.2 V) respectively
(c) (–4 mA, +0.2 V) and (2 mA, 0.2 V) respectively
(d) (–4 mA, +0.2 V) and (2 mA, 0.1 V) respectively
34. While performing the experiment on studying the dependence of current (I) on the potential
difference (V) across a resistor, four students I, II, III and IV set up the circuit is shown.
The correct result will be obtained by the student.
(a) I (b) II (c) III (d) IV
35. If the key in the arrangement is taken out (the circuit is made open) and magnetic field lines
are drawn over the horizontal plane, the lines are:
(a) concentric circles.
(b) elliptical in shape.
(c) straight lines parallel to each other.
(d) concentric circles near the point O but of elliptical shapes as we go away from it.
36. Fuel used in thermal power plants is:
(a) water (b) uranium (c) biomass (d) fossil fuels






No comments:
Post a Comment