SECTION A
Question 1:
Define photosynthesis.
Answer:
The process by which green plants make their own food (like glucose) from carbon dioxide and water by using solar energy in the presence of chlorophyll is called photosynthesis.
Question 2:Define photosynthesis.
Answer:
The process by which green plants make their own food (like glucose) from carbon dioxide and water by using solar energy in the presence of chlorophyll is called photosynthesis.
List two natural ecosystems.
Answer:
Two natural ecosystems are: Lake and Forest.
Question 3:
State two positions in which a concave mirror produces a magnified image of a given object. List two differences between the two images.
Answer:
The two positions are:
(i) When an object is placed between the pole (p) and focus ( of a concave mirror, the image formed is larger than the object (or magnified).
(ii) When an object is placed between the focus ( and centre of curvature (c) of a concave mirror, the image formed is larger than the object.
Difference between the two images
|
|
|
|
List four important properties of aluminium which are responsible for its great
demand in industry.
Answer:
Important properties of aluminium:
(i) It is a light metal.
(ii) It does not corrode as it forms a protective layer of oxide which prevents it from further oxidation.
(iii) It is a good conductor of heat and electricity.
(iv) It is used as a reducing agent in the extraction of metals from the oxide.
Question 5:
Name the plant hormones responsible for the following functions:
(i) growth of the stem
(ii) promotes cell division
(iii) wilting of leaves
(iv) inhibits growth
Answer:
| Function | Hormone responsible |
| (i) growth of the stem | Auxin / gibberellin |
| (ii) promotes cell division | Cytokinin |
| (iii) wilting of leaves | Abscisk acid |
| (iv) inhibits growth | Abscisk acid |
Name the substance oxidised and the substance reduced, and also identify the oxidising agent and reducing agents in the following reaction:
(a) 3MnO2 + 4Al —>3Mn + 2Al2O3
(b) Fe2O3 + 3CO -> 2Fe + 3CO2
(c) SO2 + 2H2S -> 3S + 2H2O
Answer:
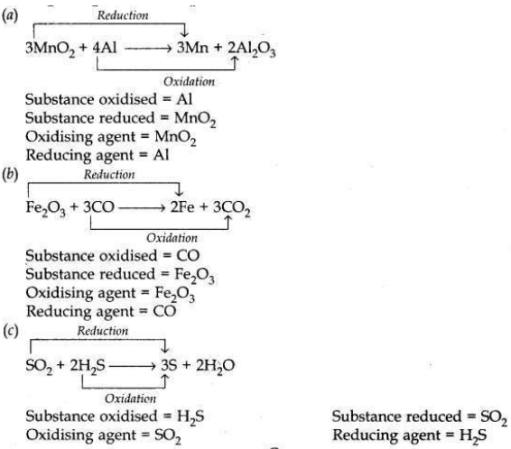
OR
Differentiate between the following with suitable examples:
(i) mineral and ore
(ii) corrosion and rancidity
(iii) malleability and ductility
Answer:
(i) Difference between Mineral and Ore
| Mineral | Ore |
| 1. The natural materials in which the metals or their compounds are found in earth are called minerals. | 1. Those minerals from which the metals can be extracted conveniently and profitably are called ores. |
| 2. Some minerals may contain only a small percentage of the metal and some may contain a large percentage of metals. | 2.An ore contains a good percentage of the metal |
| Corrosion | Rancidity |
| 1. The tarnishing of the metals by the attack of moisture and acids in the air is called corrosion. |
1. When fats and oils present in the food get oxidized, the smell and taste of the food changes. This is called Rancidity.
|
| 2. Example: Rusting of iron. | 2. Example: Potato-chips kept in N2 gas to check rancidity. |
| Malleability | Ductility |
1. The property which allows the metals to be hammered into thin sheets is called malleability.
|
1. The property which allows the metals to be drawn into thin wires is called ductility.
|
| 2. Gold and silver metals are the best malleable metals. | 2. Gold is the most ductile metal. |
Name the system which facilitates communication between central nervous system and the other parts of the body. Mention two types of nerves it consists of along with their organs of origin.
Answer:
Peripheral nervous system facilitates communication between central nervous system and the other parts of the body.
Two types of nerves:
• Cranial nerves arise from the brain and spread throughout the head.
• Spinal nerves arise from the spinal cord along most of the length of the spinal cord and spread throughout the body except the head.
Question 8:
Draw the pattern of magnetic field lines around a current carrying straight conductor. How does the strength of the magnetic field produced change:
(i) with the distance from the conductor?
(ii) with an increase in current in a conductor?
Answer:
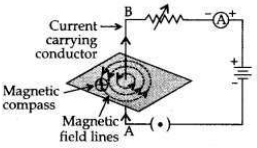
(i) Strength of the magnetic field produced by a straight current carrying wire at a point is inversely proportional to the distance of that point from the wire.
(ii) Strength of the magnetic field is directly proportional to the current passing in the wire.
Question 9:
Find the current drawn from the battery by the network of four resistors shown in the figure.
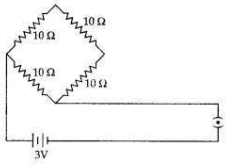
Answer:
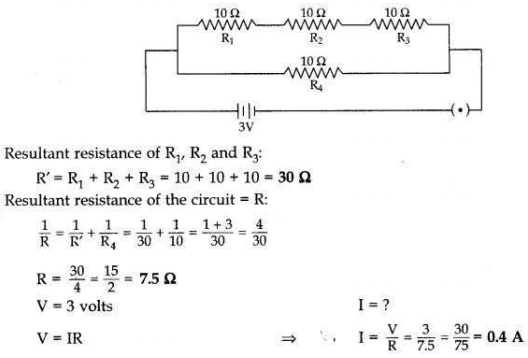
Question 10:
Anita visited her village during summer vacation and saw her grandmother burning firewood to cook food. This caused lots of smoke and resulted in the bad health of Anita’s grandmother. Anita suggested some alternatives to her family in the village and offered to help them. Now answer the following questions:
(i) List any two alternatives that Anita must have suggested to her grandmother.
(ii) How will Anita’s grandmother benefit herself and the community by not burning the firewood? Give one reason each.
(iii) Which qualities of Anita are reflected in her way of thinking?
Answer:
(i) Two alternatives suggested by Anita:
• Use of biogas
• Use of charcoal
(ii) Disadvantages of burning firewood:
• Burning of firewood causes lots of smoke which harms the person who is cooking the food and causes air pollution which is harmful for the environment and harms the society as a whole.
• Firewood leaves lots of ashes after burning so it pollutes the air we breathe thus making the atmosphere unclean.
(iii) Qualities displayed by Anita:
• Caring
• Love for her elders
• Concern for the environment
• Intelligent and has the ability to take initiative
Question 11:
What are covalent compounds? Why are they different from ionic compounds?
List their three characteristic properties.
Answer:
Covalent compounds: The chemical bond formed by the sharing of electrons between two atoms is known as a covalent bond. The molecules formed by sharing of electrons between two or more same atoms or between two or more non-metals are called covalent compounds
| Ionic bond | Covalent bond |
| 1. It is formed by transference of electrons from one atom to the other. | 1. It is formed by the sharing of electron pairs by two atoms. |
| 2. Electrostatic | 2. Not electrostatic, but rigid. |
| 3. Ionic substances are formed by ionic bonds. | 3. Molecules are formed by covalent bond. |
| 4. Non-directional. | 4. Directional. |
(i) Covalent compounds usually have low melting and boiling points as they are formed by electrically neutral molecules. So, the force of attraction between the molecules of covalent compounds is very weak. Only a small amount of heat energy is required to break these forces.
(ii) Covalent compounds are usually insoluble in water but they are soluble in organic solvents.
(iii) Covalent compounds do not conduct electricity as they do not contain ions.
Question 12:
An element ’M’ with electronic configuration (2, 8, 2) combines separately with (NO3)-, (SO4)2- and (PO4)3- radicals. Write the formula of the three compounds so formed. To which group and period of the Modern Periodic Table does the elements ’M’ belong? Will ’M’ form covalent or ionic compounds? Give reason to justify your answer.
Answer:
• The electronic configuration (2, 8, 2) of the element ’M’ suggests that it belongs to group 2 and period 3 of the Modem Periodic Table and its valency is 2.
• The chemical formulae of the compounds formed are:
(i)M(NO3)2
(ii) MSO4
(iii) M3(PO4)2
• ’M’ will form ionic compounds by losing two valence electrons to achieve a noble gas configuration, that is, a stable octet in the valence shell.
Question 13:
How do organisms, whether reproduced asexually or sexually maintain a constant chromosome number through several generations? Explain with the help of suitable example.
Answer:
• When organisms reproduce asexually, a basic event in reproduction is the creation of a DNA copy. Cells use chemical reactions to build copies of their DNA. This creates two copies of the DNA in a reproducing cell, and they will need to be separated from each other. However, keeping one copy of DNA in the original cell and simply pushing the other one out would not work, because the copy pushed out would not have any organised cellular structure for maintaining life processes. Therefore, DNA copying is accompanied by the creation of an additional cellular apparatus, and then DNA copies separate, each with its own cellular apparatus. Effectively, a cell divides to give rise to two cells. Thus, chromosome number remains unchanged. For example, reproduction of amoeba by binary fission.
• In sexual reproduction, organisms produce gametes through a special type of division, meiosis—reductional division, in which the original number of chromosomes becomes half. These two gametes then combine to form the zygote and the original number of chromosomes is restored as in the case of human beings.
OR
Suggest three contraceptive methods to control the size of human population which is essential for the health and prosperity of a country. State the basic principle involved in each.
Answer:
There are three different methods of contraception:
(i) Barrier methods. In these methods, physical devices such as condoms, diaphragms and cervical caps are used. These devices prevent the entry of sperm in the female genital tract, thus acting as a barrier between them.
(ii) Surgical methods. There are surgeries that can be carried out in males and females. In males, a small portion of the sperm duct (vas deferens) is blocked by a surgical operation. This prevents the sperms from coming out.
In females, a small portion of the fallopian tubes (oviducts) is blocked by a surgical operation. It prevents the egg from reaching the uterus. In both the cases, fertilisation will not take place.
(iii) Chemical methods. This category of contraceptives acts by changing the hormonal balance of the body so that eggs are not released and fertilisation cannot occur. Females use two types of pills for preventing pregnancies, i.e., oral pills and vaginal pills. The oral pills contain hormones which stop the ovaries from releasing ovum into the fallopian tube. This is also called oral contraceptives (OC).
Other contraceptive devices such as loop or the copper-T are placed in the uterus to prevent pregnancy.
Question 14:
Draw the following diagram, in which a ray of light is incident on a concave/convex mirror, on your answer sheet. Show the path of this ray, after reflection, in each case.
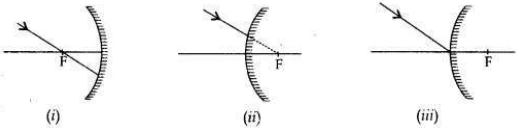
Answer:
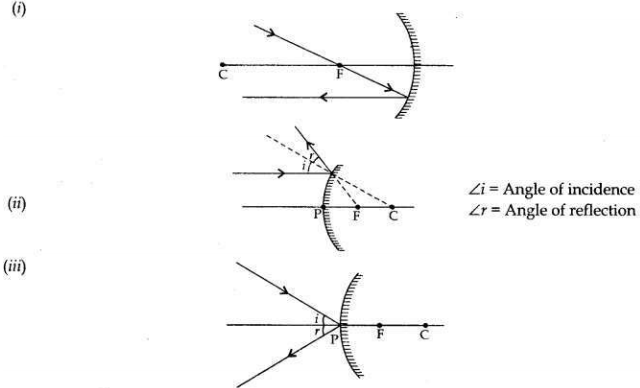
Question 15:
Why does the sun appear reddish early in the morning? Will this phenomenon be observed by an observer on the moon? Justify your answer with a reason.
Answer:
The sun at sunrise (early in the morning) is located near the horizon of the earth. Light from the sun near the horizon has to pass through thick layers of air and a large distance through the earth’s atmosphere before reaching our eyes. Near the horizon, most of the blue light rays with shorter wavelength are scattered away by the particles in the atmosphere. Therefore, the light that reaches our eyes is the red light of longer wavelengths. This gives rise to the reddish appearance of the sun.
This phenomenon will not be observed by an observer on the moon because there is no atmosphere on the moon to scatter light.
Question 16:
(a) Define a universal indicator. Mention its one use.
(b) Solution A gives pink colour when a drop of phenolphthalein indicator is added to it. Solution B gives red colour when a drop of methyl orange js added to it. What type of solutions are A and B and which one of the solutions A and B will have a higher pH value?
(c) Name one salt whose solution has pH more than 7 and one salt whose
solution has pH less than 7.
Answer:
(a) Universal indicator is a mixture of many different indicators which gives different colours at different pH values of the entire pH scale. L It shows
different colours at different concentrations of hydrogen ions in a solution.
(b) Solution A gives pink colour when a drop of phenolphthalein indicator is added, therefore A is a base.
Solution B gives red colour when a drop of methyl orange is added to it, therefore B is an acid.
Hence, solution A will have less concentration of hydrogen ion than B.
Thus, A will have pH more than 7 because pH value of:
— an acid solution < 7; — a base solution > 7; and
— a neutral solution = 7.
(c) — The salts of strong acids and weak bases give acidic solution having pH less than 7. Example, NH4Cl, Ammonium Chloride will have pH less than 7.
— The salts of a weak, acids and strong bases give basic solution having pH more than 7. Example, Na2CO3, Sodium Carbonate will have pH more than 7.
Question 17:
(a) Explain how the separation of oxygenated and deoxygenated blood is useful in humans?
(b) Why is double circulation of blood necessary in humans?
Answer:
(a) Humans have a four chambered heart which consists of two atria and two ventricles.
In a four chambered heart, the left side and right side of the heart are completely separated to prevent the oxygenated blood from mixing with deoxygenated blood. Such a separation allows a highly efficient supply of oxygen to the body cells which is necessary for producing a lot of energy. This energy is useful for a warm blooded animal (like humans) which has high energy needs to maintain body temperature.
(b) All the animals having four chambered hearts have double circulation in which the blood passes through the heart ’twice’ in one complete cycle of the body. This ensures the separation of oxygenated blood from deoxygenated blood.
Explanation:
Double circulation. The blood travels twice through the heart in one complete cycle of the body and is called double circulation. It involves two circulations:
(i) Pulmonary circulation. The pathway of the blood from the heart to the lungs and back to the heart is called pulmonary circulation. It is a small circulation. Deoxygenated blood in the right ventricle flows into the vascular system of the lungs, becomes oxygenated and returns to the heart left atrium through pulmonary veins.
(ii) Systemic circulation. The pathway of the blood from the heart to the rest of the body and back to the heart is called systemic circulation. It is a large circulation. Left ventricle sends the blood into the arota. Aorta divides into arteries, arterioles and capillaries and supplies oxygenated blood to various parts of the body. From there the deoxygenated blood is collected by venules,
which join to form veins and finally vena cava and pours blood back into right atrium.
Question 18:
For the series combination of three resistors establish the relation:
R = R1+ R2 + R3
where the symbols have their usual meanings.
Calculate the equivalent resistance of the combination of three resistors of 6 Ω , 9 Ω and 18 Ω joined in parallel.
Answer:
Same current (l) flows through different resistances, when these are joined in series, as shown in the figure.
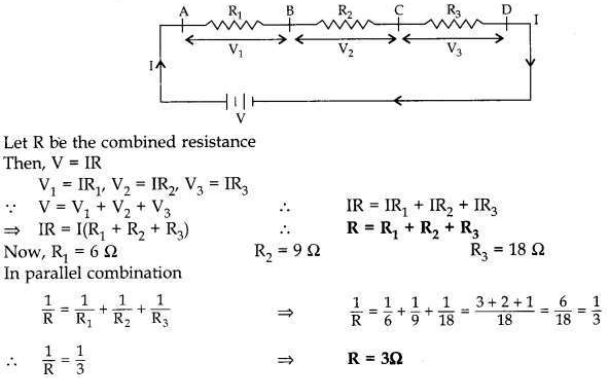
OR
What is meant by electric circuit? Why does electric current start flowing in a circuit the moment circuit is complete? When do we say that the potential difference across a conductor in a circuit is 1 volt?
Calculate the potential difference between the two terminals of a battery if 12 joules of work is done in transferring 2 coulombs of charge.
Answer:
• A continuous conducting path consisting of wires and other resistances (like electric bulb, etc.) and a switch, between the two terminals of a cell or a battery along which an electric current flows is called an electric circuit.
• It is the potential difference between the ends of the wire which makes the electric charges (or current) to flow in the wire.
The potential difference between two points is said to be 1 volt if 1 joule of
work is done in moving 1 coulomb of electric charge from one point to another.

Question 19:
(a) Give a chemical test to distinguish between saturated and unsaturated hydrocarbon.
(b) Name the products formed when ethane burns in air. Write the balanced chemical equation for the reaction showing the types of energies liberated.
(c) Why is reaction between methane and chlorine in the presence of sunlight considered a substitution reaction? 5
Answer:
(a) Bromine water test. The addition of bromine (Br2) gives addition reactions with unsaturated compounds (like alkenes and alkynes). The addition of bromine is used as a test for unsaturated compounds. For this purpose, bromine is used in the form of bromine water. A solution of bromine in water is called bromine water. Bromine water has a red-brown colour due to the presence of bromine in it. When bromine water is added to an unsaturated compound, then bromine gets added to the unsaturated compound and the red-brown colour of bromine water is discharged. So, if an organic compound decolourises bromine water, then it will be an unsaturated hydrocarbon (containing a double bond or a triple bond), but saturated hydrocarbon (alkanes) do not decolourise bromine water.
(b) When ethane burns in air, carbon dioxide and water vapours are formed along with heat and light.
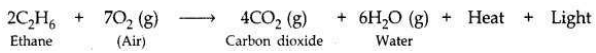
This reaction is an exothermic reaction due to the evolution of heat.
(c) Methane reacts with chlorine in the presence of sunlight to form chloromethane and hydrogen chloride.
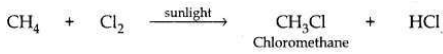
In this reaction, one ’H’ atom of methane has been substituted (replaced) by a ’Cl’ atom, converting CH4 to CH3Cl. Hence, it is considered a substitution reaction.
Question 20:
What is meant by speciation? List four factors that could lead to speciation. Which of these cannot be a major factor in the speciation of a self-pollinating plant species. Give reason to justify your answer.
Answer:
Speciation. The process by which new species develop from the existing species by evolution or any genetic modification of previous species is known as speciation.
The important factors which could lead to the formation of new species are:
(i) Geographical isolation of a population caused by various types of barriers like mountain ranges, rivers, sea etc. The geographical isolation leads to reproductive isolation due to which there is no flow of genes between separated groups of population.
(ii) Genetic drift caused by drastic changes in the frequencies of particular genes by chance alone.
(iii) Variations caused in individuals due to natural selection.
(iv) Drastic change in the genes or DNA called mutation is also a cause of speciation. Reproductive isolation cannot be a major factor for the speciation of a self-pollinating plant species as it does not depend on any other plant for its reproduction process.
Question 21:
(a) Write the function of each of the following parts of human eye: cornea; iris; crystalline lens; ciliary muscles (b) Millions of people of the developing countries of the world are suffering from corneal blindness. These persons can be cured by replacing the defective cornea with the cornea of a donated eye. A charitable society of your city has organised a campaign in your neighbourhood in order to create awareness about this fact. If you are asked to participate in this mission how would you contribute in this noble cause?
(i) State the objective of organising such campaigns.
(ii) List two arguments which you would give to motivate the people to donate their eyes after death.
(iii) List two values which are developed in the persons who actively participate and contribute in such programmes.
Answer:
(a) Functions of the following parts of human eye:
(i) Cornea. The front part of the eye is called cornea. It is made up of a transparent substance. The light coming from objects enters the eye through cornea.
(ii) Iris. This is a flat, coloured, ring-shaped membrane behind the cornea. Pupil is a hole in the middle of the iris. Iris controls the size of the pupil.
(iii) Crystalline lens. Eye lens is a convex lens which focuses the image of the object on the retina.
(iv) Ciliary muscles. Ciliary muscles hold the eye lens and changes the thickness of eye-lens while focussing.
(b) (i) Objective of such campaigns. To make people aware of corneal blindness and make them realise their duties towards the society by taking pledge for eye donation.
(ii) • One pair of eyes can give eyesight to two corneal blind persons (each getting one eye), and make them see this beautiful world.
• Our eyes can live even after our death. People belonging to all age groups, even people with medical conditions like cataract, diabetes, hypertension can donate their eyes.
(iii) Values developed in persons who actively participate in such programmes:
• Concern for others and social welfare.
• Responsible behaviour and awareness.
SECTION B
Question 22:While studying the double displacement reaction, the solutions of barium chloride and sodium sulphate are mixed together. 2
(i) What do you observe as soon as the two solutions are mixed together?
(ii) What will happen in the above observation made by you after ten minutes?
Answer:
(i) The reaction mixture becomes white in colour and a precipitate is formed.
(ii) White precipitate settles down after 10 minutes.
Question 23:
In an experiment, to study the dependence of potential difference (V) on the electric current (I) across a conductor (resistor), if the circuit is on for long time, then—select two correct options from the following:
(i) Zero error of an ammeter will be changed.
(ii) Zero error of a voltmeter will be changed.
(iii) Value of a resistance will be changed.
(iv) Resistor will be heated.
Answer:
(i) Value of resistance will be changed.
(ii) Resistor will be heated.
Question 24:
Record your observations when a stained and mounted leaf peel is viewed by
you under high power (45 x) microscope.
Answer:
(i) The process (stomatal process) in which stomatal pores are clearly seen.
(ii) Each stomata has two kidney shaped cells (guard cells) having one nucleus and many chloroplasts each.
Question 25:
What do you observe when you drop a few drops of acetic acid to a test tube
containing:
(i) phenolphthalein
(ii) distilled water
(iii) universal indicator
(iv) sodium hydrogen carbonate powder
Answer:
Action of acetic acid on
(i) phenolphthalein → no change/remains colourless
(ii) distilled water → no change, acetic acid dissolves in distilled water.
(iii) universal indicator → Turns orange
(iv) sodium hydrogen carbonate powder → evolution of colourless, odourless gas with brisk effervescence.
Question 26:
Draw a labelled diagram to show that particular stage of binary fission in amoeba in which its nucleus elongates and divides into two and a constriction appears in its cell membrane.
Answer:
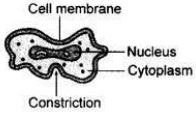
Question 27:
A student focuses the image of a well illuminated distant object on a screen using a convex lens. After that he gradually moves the object towards the lens and each time focuses its image on the screen by adjusting the lens.
(a) In which direction—towards the screen or away from the screen, does he move the lens?
(b) What happens to the size of the image—does it decrease or increase?
(c) What happens to the image on the screen when he moves the object very close to
the lens?
Answer:
(a) He should move the lens away from the screen.
(b) Size of the image increases.
(c) No image will be formed on the screen



Thanks for the useful blog, I found it informative, hope for more blogs like this from you.
ReplyDeleteIf you are looking for the Best Schools in Delhi then you are at the right place. GD GOENKA Rohini is the best CBSE School in Delhi. Now admissions are open in 2021-2022
Thank you for such valuable information about CBSE Class 10th Science Sample Paper 2019, if you would like to know more about best schools of CBSE Board, visit Cbse Schools Bangalore
ReplyDelete