CBSE Class 10th Science Sample paper 2019 part 6
Download Solved CBSE Sample Papers for Class 10 Science Set 6 2019 PDF to understand the pattern of questions asks in the board exam. Know about the important topics and questions to be prepared for CBSE Class 10 Science board exam and Score More marks. Here we have given Science Sample Paper for Class 10 Solved Set 6.
Board – Central Board of Secondary Education, cbse.nic.in
Subject – CBSE Class 10 Science
Year of Examination – 2019
Mention the purpose of blackening the interior of a solar cooker.
Answer:
The black painted surface is used in the interior of a solar cooker because
black surface absorbs more heat rays of the sun.
Question 2:
Write the number of covalent bonds in the molecule of ethane.
Answer:

Question 3:
The absolute refractive indices of glass and water are 3/2 and 4/3 respectively. If the speed of light is 2 x 108 m/s, calculate the speed of light in
(i) vacuum,
(ii) water.
Answer:
SECTION A
Question 1:Mention the purpose of blackening the interior of a solar cooker.
Answer:
The black painted surface is used in the interior of a solar cooker because
black surface absorbs more heat rays of the sun.
Question 2:
Write the number of covalent bonds in the molecule of ethane.
Answer:

Question 3:
The absolute refractive indices of glass and water are 3/2 and 4/3 respectively. If the speed of light is 2 x 108 m/s, calculate the speed of light in
(i) vacuum,
(ii) water.
Answer:
CBSE Class 10th Science Sample Paper 2019 part 5
Download Solved CBSE Sample Papers for Class 10 Science Set 5 2019 PDF to understand the pattern of questions asks in the board exam. Know about the important topics and questions to be prepared for CBSE Class 10 Science board exam and Score More marks. Here we have given Science Sample Paper for Class 10 Solved Set 5.
Name the two components of peripheral nervous system.
Answer:
Two components of peripheral nervous system:
What is the function of ozone in the upper atmosphere?
Answer:
Ozone layer is very important for the existence of life on earth. The function of the ozone layer in the upper atmosphere is to absorb most of the harmful ultraviolet radiations coming from the sun and prevent them from reaching the earth’s surface.
Question 3:
List four characteristics of the images formed by plane mirrors.
Answer:
The characteristics of the images formed by plane mirrors are:
When hydrogen gas is passed over heated copper (II) oxide, copper and steam are formed. Write the balanced chemical equation for this reaction and state (i) the substance oxidized and (ii) the substance reduced in the reaction.
Answer:
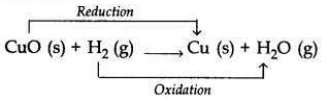
(i) Substance oxidized = H2 (Hydrogen gas)
(ii) Substance reduced = CuO (Copper oxide)
Question 5:
What is meant by “sustainable management”? Why is reuse considered better than recycling?
Answer:
The development and management of resources in such a way which meets the current basic human needs and also preserves the resources for the needs of future generations, is called sustainable management.
The process of ‘reuse’ is considered better than the process of ‘recycling’ because recycling requires the use of a large amount of energy and money whereas no energy is required for reusing materials.
Question 6:
State reason for the following:
(i) Lemon is used for restoring the shine of tarnished copper vessels.
(ii) A metal sulphide is converted into its oxide to extract the metal from the sulphide ore.
(iii) Copper wires are used in electrical connections.
Answer:
(i) When a copper object remains in damp air for a considerable time, then copper reacts slowly with carbon dioxide and water in air to form a green coating of basic copper carbonate on its surface. If corroded copper vessels are treated with lemon which is acidic in nature, the acid solution dissolves green coloured basic copper carbonate and makes them look shiny.
(ii) It is easier to obtain metals from their oxides (by reduction) than from sulphides. So before reduction, the metal sulphide ore is converted into metal oxide.
(iii) Copper metal is the next best conductor of electricity after silver metal. So electric wires are made of copper (as silver being a costly metal can not be used for making electric wires).
Question 7:
State the kind of chemical reactions in the following examples:
(i) Digestion of food in stomach
(ii) Combustion of coal in air
(iii) Heating of limestone
Answer:
(i) Digestion of food in stomach. During digestion, the complex food is broken into simpler form. Therefore, it is a type of decomposition reaction.
(ii) Combustion of coal is air. During combustion the coal bums in air to form CO2, H2O along with the evolution of heat. Thus, it is a type of exothermic decomposition reaction.
(iii) Heating of limestone. When limestone is heated strongly, it breaks into CO2 and lime. Thus, it is a type of thermal decomposition reaction.
Question 8:
The rate of breathing in aquatic organisms is much faster than that seen in terrestrial organisms. Give reason. State the pathway of air from nostrils to the lungs in human beings.
Answer:
The animals which live in water (aquatic animals) use the oxygen dissolved in water to carry out respiration. Since the amount of dissolved oxygen in water is low as compared to the amount of oxygen in the air, therefore, the rate of breathing in aquatic animals is much faster than in terrestrial animals. A faster rate of breathing provides more oxygen to aquatic animals.
Pathway of air in human beings:
Nostrils → Pharynx → Larynx → Trachea → Lungs
OR
Mention three characteristic features of hormonal secretions in human beings.
Answer:
(i) A group of endocrine glands which produces various hormones is called an endocrine system. The endocrine system is also called hormonal system.
(ii) The endocrine system also helps in coordinating the activities of our body. The endocrine system in our body consists of a number of glands (or tissues) which make, store, and release chemicals called hormones.
(iii) The working of endocrine glands is controlled by our nervous system. The hormones produced by endocrine glands act as messengers between the nervous system and the organs of our body.
Question 9:
A circuit has la line of 5 A. How many lamps of rating 10W; 220V can simultaneously run on this line safely?
Answer:

Question 10:
Amit lives in Delhi and is much concerned about the increasing electricity bill of his house. He took some steps to save electricity and succeeded in doing so.
(i) Mention any two steps that Amit might have taken to save electricity.
(ii) Amit fulfilled his duty towards the environment by saving electricity. How?
(iii) Which alternative source of energy would you suggest Amit to use?
Answer:
(i)
• He can use energy efficient electrical appliances to save electricity. This can be done by using CFL bulbs and tube lights in place of conventional filament type electric bulbs.
• Use solar water heater instead of electrical geyser.
(ii) By saving electricity, Amit is contributing in his small way towards reducing environmental degradation. Most of the electrical appliances use electrical energy which is generated by burning fossil fuel. The burning of fossil fuels causes air pollution. The production of hydroelectricity causes ecological imbalance. Therefore, by using less electricity he is indirectly causing less pollution.
(iii) He can use solar energy devices like solar cooker, solar water heater and solar cells. With the help of an example, explain the process of hydrogenation.
Question 11:
With the help of an example, explain the process of hydrogenation Mention the essential conditions for the reaction and state the change in physical property with the formation of the product.
Answer:
Process of hydrogenation:
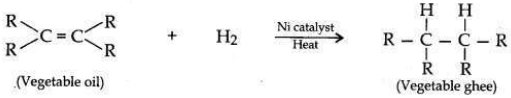
The addition of hydrogen to an unsaturated hydrocarbon to obtain a saturated hydrocarbon is called hydrogenation.
Essential conditions for the reaction are:
(i) Presence of an unsaturated hydrocarbon.
(ii) Presence of a catalyst such as nickel (Ni) or palladium.
Changes observed:
• Change observed in the physical property is the change of unsaturated compound from the liquid state to saturated compound in solid state.
• The boiling or melting points of a product is increased.
Question 12:
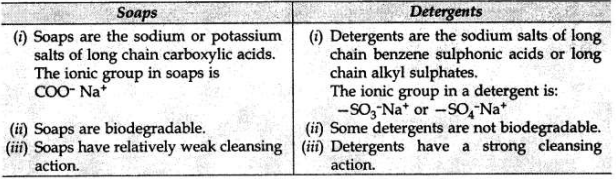
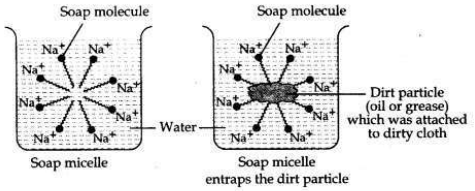
What is the difference between the molecules of soaps and detergents, chemically? Explain the cleansing action of soaps.
Answer:
Difference between molecules of soaps and detergents
Action of soap in removing an oily spot from a piece of cloth. Soaps are molecules in which the two ends have differing properties, one is hydrophilic, that is, it dissolves in water, while the other end is hydrophobic, that is, it dissolves in hydrocarbons. When soap is at the surface of water, the hydrophobic ’tail’ of soap will not be soluble in water and the soap will align along the surface of water with the ionic end in water and the hydrocarbon ’tail’ protruding out of water. Inside water, these molecules have a unique orientation that keeps the hydrocarbon portion out of the water.
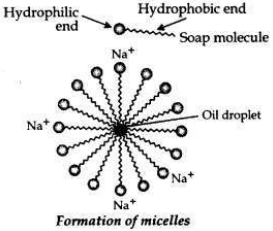
This is achieved by forming clusters of molecules in which the hydrophobic tails are in the interior of the cluster and the ionic ends are on the surface of the cluster. This formation is called a micelle. Soap in the form of a micelle is able to clean, since the oily dirt will be collected in the centre of the micelle. The micelles stay in solution as a colloid and will not come together to precipitate because of ion-ion repulsion. Thus, the dirt suspended in the micelles is also easily rinsed away.
OR
How many groups and periods are there in the modem periodic table? How do the atomic size and metallic character of elements vary as we move:
(i) down a group and (ii) from left to right in a period?
Solution:
There are 18 groups and 7 periods in the modem periodic table.
(i) Atomic size and metallic character of the elements increases down a group.
(ii) • Atomic size and metallic character of elements decreases from left to right in a period.
• Metallic character of the element is decreased.
Question 13:
What is DNA copying? State its importance.
Answer:
A process in which a DNA molecule produces two similar copies of itself in a reproducing cell through chemical reaction is a called DNA copying.
(i) It transmits the characteristics from parents to the next generation (offspring).
(ii) It causes increased variations in the population.
Question 14:
To construct a ray diagram we use two rays of light which are so chosen that it is easy to determine their directions after reflection from the mirror. Choose these two rays and state the path of these rays after reflection from a concave mirror. Use these two rays to find the nature and position of the image of an object placed at a distance of 15 cm from a concave mirror of focal length 10 cm.
Answer:
Ray 1. When an incident ray of light is parallel to the principal axis of a concave mirror, its reflected ray must pass through the principal focus of the concave mirror.
Ray 2. A ray passing through the principal focus emerge parallel to the principal axis
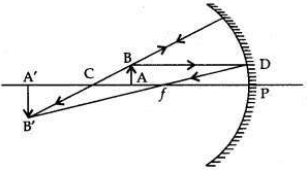
Focal length = 10 cm;
Then centre of curvature, C = 20 cm Object is placed at 15 cm, i.e., between F & C When the object is between F and C (centre of curvature):
The image formed is real, inverted and magnified. It is formed beyond C.
Question 15:
With the help of a labelled diagram, explain why the sun appears reddish at the sunrise and the sunset.
Answer:
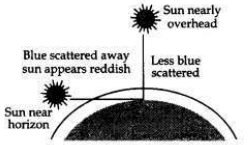
At the time of sunrise and sunset when the sun is near the horizon, the sunlight has to travel the greatest distance through the atmosphere to reach us. During the long journey of sunlight, most of the shorter wavelength blue-colour present in it is scattered out and away from our line of sight so, the light reaching us directly from the rising sun or setting sun consists mainly of longer wavelength red colour due to which the sun appears red.
Due to the same reason, the sky surrounding the rising sun and setting sun also appears red. Thus, at sunrise and sunrise, the sun itself as well as the surrounding sky appear red.
Question 16:
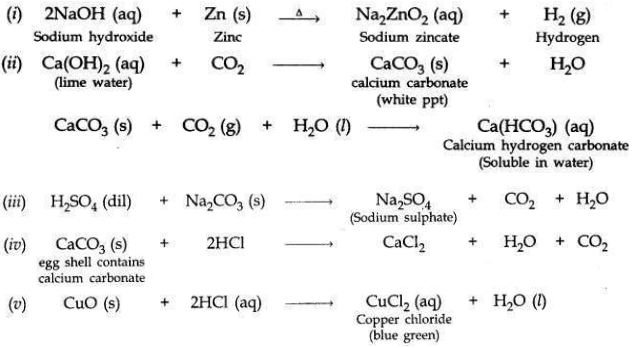
Write balanced chemical equations for the following statements:
(i) NaOH solution is heated with zinc granules.
(ii) Excess of carbon dioxide gas is passed through lime water.
(iii) Dilute sulphuric acid reacts with sodium carbonate.
(iv) Egg shells are dropped in hydrochloric acid.
(v) Copper (II) oxide reacts with dilute hydrochloric acid.
Answer:
Question 17:
(a) Write three main functions of the nervous system.
(b) In the absence of muscle cells, how do plant cells show movement?
Answer:
(a) Main functions of the nervous system:
• Coordinate the activities of the body.
• Helps all other systems of the body to work together.
• The nervous system receives information from the surroundings, processes it, interprets it and then responds accordingly.
(b) The movement in any part of a plant is usually a growth movement or change in shape of body parts.
(a) Draw magnetic field lines of a bar magnet. “Two magnetic field lines never intersect each other.” Why?
(b) An electric oven of 1.5 kW is operated in a domestic circuit (220 V) that has a current rating of 5 A. What result do you expect in this case? Explain.
Answer:
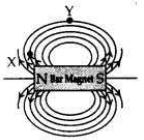
(a) Two magnetic field lines do not intersect one another. The direction of magnetic field lines is always from north pole to south pole. If the two magnetic field lines do intersect, it means at the point of intersection the compass needle
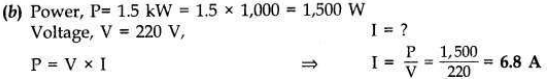
Now the current drawn by the oven is 6.8 A which is very high but the fuse in this circuit is only 5 A capacity. When a very high current of 6.8 A flows through 5 A fuse, the fuse wire will get heated too much, melt and break the circuit, cutting off the power supply.
OR
What is meant by resistance of a conductor? Name and define its SI unit. List the factors on which the resistance of a conductor depends. How is the resistance of a wire affected if —
(i) its length is doubled,
(ii) its radius is doubled?
Answer:
The property of a conductor due to which tends to stop the flow of current through the conductor is called resistance.
SI unit is ohm. When a potential difference of IV across a wire gives rise to IA current through the wire, then the resistance is said to be 1 ohm (1 Q).
The resistance of conductor depends on length, thickness, nature of material and temperature of conductor.
(i) If length is doubled, then R is doubled because the resistance of a conductor is directly proportional to length.
(ii) Resistance of a conductor is inversely proportional to the square of its diameter or area of cross-section.
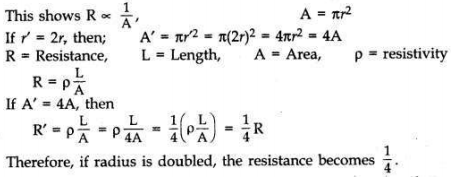
Question 19:
Explain why carbon forms compounds mainly by covalent bond. Explain in brief two main reasons for carbon forming a large number of compounds. Why does carbon form strong bonds with most other elements?
Answer:

Since, carbon has 4 electrons in its outermost shell so, it should either lose or gain 4 electrons to achieve the inert gas configuration and become stable.
(i) It could gain four electrons forming C4- anion. But it would be difficult for the nucleus with six protons to hold on to ten electrons due to inter electronic repulsion.
(ii) It could lose 4 electrons forming C4+ cation. But it would require a large amount of energy to remove four electrons from its outermost shell.
Thus, it forms compounds mainly by covalent bonds.
Two properties of carbon which lead to huge number of carbon compounds are:
(i) Catenation. Catenation is the unique property of carbon atoms to form bonds with other atoms of carbon giving rise to large molecules.
(ii) Tetravalency. Since carbon has a valency of 4, it is capable of bonding with 4 other atoms of carbon or atoms of some other monovalent element.
The reason for the formation of strong bonds by the carbon atoms is their small atomic size. Due to the small size of carbon atoms their nuclei hold the shared pairs of electrons between atoms strongly, leading to the formation of strong covalent bonds. The carbon atoms also form strong covalent bonds with the atoms of other elements such as hydrogen, oxygen, nitrogen, sulphur, chlorine and other elements.
Question 20:
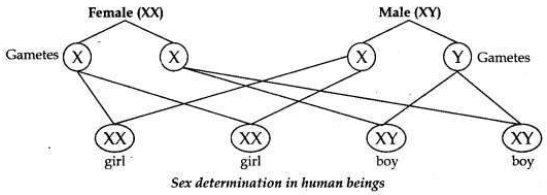
How many pairs of chromosomes are present in human beings? Out of these how many are sex chromosomes? How many types of sex chromosomes are found in human beings? “The sex of a newborn child is a matter of chance and none of the parents may be considered responsible for it”. Draw a flow chart showing determination of sex of a newborn to justify this statement.
Answer:
• There are 23 pairs of chromosomes present in human beings.
• There is 1 pair of sex chromosomes present in human beings.
• The chromosomes which determine the sex of a person are called sex chromosomes. There are two types of sex chromosomes, one is called X chromosome and the other is called Y chromosome. Males contain one X chromosome and one Y chromosome (XY), while females contain two X chromosomes (XX).
Flow chart showing determination of sex of a child.
Therefore it is the sperm from the father which determines the sex of the child.
Question 21:
“A convex lens can form a magnified erect as well as magnified inverted image of an object placed in front of it.” Draw ray diagram to justify this statement stating the position of the object with respect to the lens in each case.
An object of height 4 cm is placed at a distance of 20 cm from a concave lens of focal length 10 cm. Use lens formula to determine the position of the image formed. 5 Answer:
Answer:
• A convex lens can form a magnified erect image when the object is placed between the optical centre and principal focus of the convex lens (i.e., between O and F’).
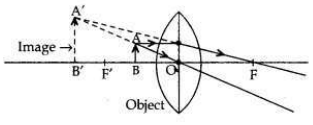
• A convex lens can form a magnified inverted image when the object is placed between focus and the centre of curvature (i.e., between F’ and 2F’).
• Object height, n = 4 cm;
Object distance, u = -20 cm Nature of the lens = concave lens; Focal length = f = -10 cm Image distance, v = ?
According to the lens formula,

So, image is formed at 6.66 cm from lens on same side. Image is erect, virtual and diminished in size.
An iron nail is dipped in the solution of copper sulphate for about 30 minutes, state the change in colour observed. Give the reason for the change.
Answer:
Iron is more reactive than Cu. So it displaces Cu from copper sulphate solution. Thus, blue colour of the CuS04 solution fades and colour of the solution turns green due to the formation of ferrous sulphate Solution. This is a displacement reaction.
CuSO4 (aq) + Fe (s) —> FeSO4 (aq) + Cu (s)
(Blue) (Green)
Question 23:
A student while verifying Ohm’s law calculated the value of resistance of the resistor for each set of observation. However, the values of resistance were slightly different from the actual value. Is his experiment wrong? Justify your answer.
Answer:
His experiment is correct since some of the current is utilized to overcome the resistance of the wires of the circuit and instruments like ammeter and
voltmeter. Thus the experimental values of resistance were different from the actual value of resistance.
Question 24:
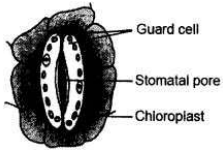
Draw a labelled diagram of stomatal apparatus with closed stomatal pore.
Answer:
Question 25:
List two observations which you make when you add a pinch of sodium hydrogen carbonate to acetic acid in a test tube. Write the chemical equation for the reaction that occurs.
Answer:
When a pinch of sodium hydrogen carbonate is added to acetic acid in a test tube, there are two observations:
(i) Brisk effervescence
(ii) Evolution of a colourless and odourless gas which is CO2.
CH3 COOH (aq) + NaHCO3 (s) -> CH3 COONa (aq) + H2O (I) + CO2 (g) ↑
Question 26:
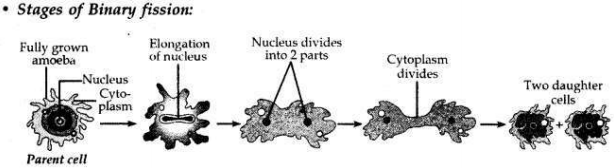
Name the type of asexual reproduction in which two individuals are formed from a single parent and the parental identity is lost.
Draw the initial and the final stages of this type of reproduction. State the event with which this reproduction starts.
Answer:
• Binary fission is a type of asexual reproduction in which two individuals are formed from a single parent and the parental identity is lost.
• This reproduction starts with the elongation of the nucleus.
Question 27:
To find the image-distance for varying object-distances in case of a convex
lens, a student obtains on a screen a sharp image of a bright object placed very far from the lens. After that he gradually moves the object towards the lens and each time focuses
(a) In which direction-towards or away from the lens, does he move the screen to focus the object?
(b) What happens to the size of image—does it increase or decrease?
(c) What happen when he moves the object very close to the lens?
Answer:
fa) He moves the screen away from iens to focus the object, l b) The size of the image increases.
Ic) When the object is placed very close to the lens, then no image will be formed on the screen. As now image formed is erect and virtual.
SECTION A
Question 1:Name the two components of peripheral nervous system.
Answer:
Two components of peripheral nervous system:
- Autonomic nervous system.
- Voluntary nervous system.
What is the function of ozone in the upper atmosphere?
Answer:
Ozone layer is very important for the existence of life on earth. The function of the ozone layer in the upper atmosphere is to absorb most of the harmful ultraviolet radiations coming from the sun and prevent them from reaching the earth’s surface.
Question 3:
List four characteristics of the images formed by plane mirrors.
Answer:
The characteristics of the images formed by plane mirrors are:
(i) The image formed by a plane mirror is virtual and erect. It cannot be received on a screen.
(ii) The image formed by a plane mirror is of the same size as the object.
(iii) The image formed by a plane mirror is at the same distance behind the mirror as the object is in front of the mirror.
(iv) The image formed in a plane mirror is laterally inverted.
Question 4:(ii) The image formed by a plane mirror is of the same size as the object.
(iii) The image formed by a plane mirror is at the same distance behind the mirror as the object is in front of the mirror.
(iv) The image formed in a plane mirror is laterally inverted.
When hydrogen gas is passed over heated copper (II) oxide, copper and steam are formed. Write the balanced chemical equation for this reaction and state (i) the substance oxidized and (ii) the substance reduced in the reaction.
Answer:

(i) Substance oxidized = H2 (Hydrogen gas)
(ii) Substance reduced = CuO (Copper oxide)
Question 5:
What is meant by “sustainable management”? Why is reuse considered better than recycling?
Answer:
The development and management of resources in such a way which meets the current basic human needs and also preserves the resources for the needs of future generations, is called sustainable management.
The process of ‘reuse’ is considered better than the process of ‘recycling’ because recycling requires the use of a large amount of energy and money whereas no energy is required for reusing materials.
Question 6:
State reason for the following:
(i) Lemon is used for restoring the shine of tarnished copper vessels.
(ii) A metal sulphide is converted into its oxide to extract the metal from the sulphide ore.
(iii) Copper wires are used in electrical connections.
Answer:
(i) When a copper object remains in damp air for a considerable time, then copper reacts slowly with carbon dioxide and water in air to form a green coating of basic copper carbonate on its surface. If corroded copper vessels are treated with lemon which is acidic in nature, the acid solution dissolves green coloured basic copper carbonate and makes them look shiny.
(ii) It is easier to obtain metals from their oxides (by reduction) than from sulphides. So before reduction, the metal sulphide ore is converted into metal oxide.
(iii) Copper metal is the next best conductor of electricity after silver metal. So electric wires are made of copper (as silver being a costly metal can not be used for making electric wires).
Question 7:
State the kind of chemical reactions in the following examples:
(i) Digestion of food in stomach
(ii) Combustion of coal in air
(iii) Heating of limestone
Answer:
(i) Digestion of food in stomach. During digestion, the complex food is broken into simpler form. Therefore, it is a type of decomposition reaction.
(ii) Combustion of coal is air. During combustion the coal bums in air to form CO2, H2O along with the evolution of heat. Thus, it is a type of exothermic decomposition reaction.
(iii) Heating of limestone. When limestone is heated strongly, it breaks into CO2 and lime. Thus, it is a type of thermal decomposition reaction.
Question 8:
The rate of breathing in aquatic organisms is much faster than that seen in terrestrial organisms. Give reason. State the pathway of air from nostrils to the lungs in human beings.
Answer:
The animals which live in water (aquatic animals) use the oxygen dissolved in water to carry out respiration. Since the amount of dissolved oxygen in water is low as compared to the amount of oxygen in the air, therefore, the rate of breathing in aquatic animals is much faster than in terrestrial animals. A faster rate of breathing provides more oxygen to aquatic animals.
Pathway of air in human beings:
Nostrils → Pharynx → Larynx → Trachea → Lungs
OR
Mention three characteristic features of hormonal secretions in human beings.
Answer:
(i) A group of endocrine glands which produces various hormones is called an endocrine system. The endocrine system is also called hormonal system.
(ii) The endocrine system also helps in coordinating the activities of our body. The endocrine system in our body consists of a number of glands (or tissues) which make, store, and release chemicals called hormones.
(iii) The working of endocrine glands is controlled by our nervous system. The hormones produced by endocrine glands act as messengers between the nervous system and the organs of our body.
Question 9:
A circuit has la line of 5 A. How many lamps of rating 10W; 220V can simultaneously run on this line safely?
Answer:

Question 10:
Amit lives in Delhi and is much concerned about the increasing electricity bill of his house. He took some steps to save electricity and succeeded in doing so.
(i) Mention any two steps that Amit might have taken to save electricity.
(ii) Amit fulfilled his duty towards the environment by saving electricity. How?
(iii) Which alternative source of energy would you suggest Amit to use?
Answer:
(i)
• He can use energy efficient electrical appliances to save electricity. This can be done by using CFL bulbs and tube lights in place of conventional filament type electric bulbs.
• Use solar water heater instead of electrical geyser.
(ii) By saving electricity, Amit is contributing in his small way towards reducing environmental degradation. Most of the electrical appliances use electrical energy which is generated by burning fossil fuel. The burning of fossil fuels causes air pollution. The production of hydroelectricity causes ecological imbalance. Therefore, by using less electricity he is indirectly causing less pollution.
(iii) He can use solar energy devices like solar cooker, solar water heater and solar cells. With the help of an example, explain the process of hydrogenation.
Question 11:
With the help of an example, explain the process of hydrogenation Mention the essential conditions for the reaction and state the change in physical property with the formation of the product.
Answer:
Process of hydrogenation:
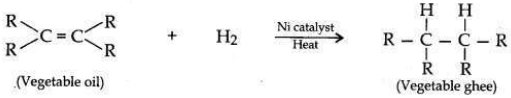
The addition of hydrogen to an unsaturated hydrocarbon to obtain a saturated hydrocarbon is called hydrogenation.
Essential conditions for the reaction are:
(i) Presence of an unsaturated hydrocarbon.
(ii) Presence of a catalyst such as nickel (Ni) or palladium.
Changes observed:
• Change observed in the physical property is the change of unsaturated compound from the liquid state to saturated compound in solid state.
• The boiling or melting points of a product is increased.
Question 12:
What is the difference between the molecules of soaps and detergents, chemically? Explain the cleansing action of soaps.
Answer:
Difference between molecules of soaps and detergents

Action of soap in removing an oily spot from a piece of cloth. Soaps are molecules in which the two ends have differing properties, one is hydrophilic, that is, it dissolves in water, while the other end is hydrophobic, that is, it dissolves in hydrocarbons. When soap is at the surface of water, the hydrophobic ’tail’ of soap will not be soluble in water and the soap will align along the surface of water with the ionic end in water and the hydrocarbon ’tail’ protruding out of water. Inside water, these molecules have a unique orientation that keeps the hydrocarbon portion out of the water.

This is achieved by forming clusters of molecules in which the hydrophobic tails are in the interior of the cluster and the ionic ends are on the surface of the cluster. This formation is called a micelle. Soap in the form of a micelle is able to clean, since the oily dirt will be collected in the centre of the micelle. The micelles stay in solution as a colloid and will not come together to precipitate because of ion-ion repulsion. Thus, the dirt suspended in the micelles is also easily rinsed away.

OR
How many groups and periods are there in the modem periodic table? How do the atomic size and metallic character of elements vary as we move:
(i) down a group and (ii) from left to right in a period?
Solution:
There are 18 groups and 7 periods in the modem periodic table.
(i) Atomic size and metallic character of the elements increases down a group.
(ii) • Atomic size and metallic character of elements decreases from left to right in a period.
• Metallic character of the element is decreased.
Question 13:
What is DNA copying? State its importance.
Answer:
A process in which a DNA molecule produces two similar copies of itself in a reproducing cell through chemical reaction is a called DNA copying.
(i) It transmits the characteristics from parents to the next generation (offspring).
(ii) It causes increased variations in the population.
Question 14:
To construct a ray diagram we use two rays of light which are so chosen that it is easy to determine their directions after reflection from the mirror. Choose these two rays and state the path of these rays after reflection from a concave mirror. Use these two rays to find the nature and position of the image of an object placed at a distance of 15 cm from a concave mirror of focal length 10 cm.
Answer:
Ray 1. When an incident ray of light is parallel to the principal axis of a concave mirror, its reflected ray must pass through the principal focus of the concave mirror.
Ray 2. A ray passing through the principal focus emerge parallel to the principal axis

Focal length = 10 cm;
Then centre of curvature, C = 20 cm Object is placed at 15 cm, i.e., between F & C When the object is between F and C (centre of curvature):
The image formed is real, inverted and magnified. It is formed beyond C.
Question 15:
With the help of a labelled diagram, explain why the sun appears reddish at the sunrise and the sunset.
Answer:

At the time of sunrise and sunset when the sun is near the horizon, the sunlight has to travel the greatest distance through the atmosphere to reach us. During the long journey of sunlight, most of the shorter wavelength blue-colour present in it is scattered out and away from our line of sight so, the light reaching us directly from the rising sun or setting sun consists mainly of longer wavelength red colour due to which the sun appears red.
Due to the same reason, the sky surrounding the rising sun and setting sun also appears red. Thus, at sunrise and sunrise, the sun itself as well as the surrounding sky appear red.
Question 16:
Write balanced chemical equations for the following statements:
(i) NaOH solution is heated with zinc granules.
(ii) Excess of carbon dioxide gas is passed through lime water.
(iii) Dilute sulphuric acid reacts with sodium carbonate.
(iv) Egg shells are dropped in hydrochloric acid.
(v) Copper (II) oxide reacts with dilute hydrochloric acid.
Answer:

Question 17:
(a) Write three main functions of the nervous system.
(b) In the absence of muscle cells, how do plant cells show movement?
Answer:
(a) Main functions of the nervous system:
• Coordinate the activities of the body.
• Helps all other systems of the body to work together.
• The nervous system receives information from the surroundings, processes it, interprets it and then responds accordingly.
(b) The movement in any part of a plant is usually a growth movement or change in shape of body parts.
- The movements of the plant part are usually caused by an unequal growth in its two regions by the action of plant hormones, under the influence of the stimuli like light, force of gravity, chemical substances, water, touch etc.
- The change in shape occurs by changing the amount of water in the body part. Water causes swelling and shrinking which causes movement.
(a) Draw magnetic field lines of a bar magnet. “Two magnetic field lines never intersect each other.” Why?
(b) An electric oven of 1.5 kW is operated in a domestic circuit (220 V) that has a current rating of 5 A. What result do you expect in this case? Explain.
Answer:

(a) Two magnetic field lines do not intersect one another. The direction of magnetic field lines is always from north pole to south pole. If the two magnetic field lines do intersect, it means at the point of intersection the compass needle
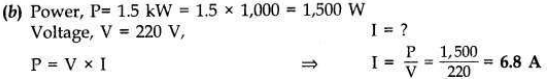
Now the current drawn by the oven is 6.8 A which is very high but the fuse in this circuit is only 5 A capacity. When a very high current of 6.8 A flows through 5 A fuse, the fuse wire will get heated too much, melt and break the circuit, cutting off the power supply.
OR
What is meant by resistance of a conductor? Name and define its SI unit. List the factors on which the resistance of a conductor depends. How is the resistance of a wire affected if —
(i) its length is doubled,
(ii) its radius is doubled?
Answer:
The property of a conductor due to which tends to stop the flow of current through the conductor is called resistance.
SI unit is ohm. When a potential difference of IV across a wire gives rise to IA current through the wire, then the resistance is said to be 1 ohm (1 Q).
The resistance of conductor depends on length, thickness, nature of material and temperature of conductor.
(i) If length is doubled, then R is doubled because the resistance of a conductor is directly proportional to length.
(ii) Resistance of a conductor is inversely proportional to the square of its diameter or area of cross-section.
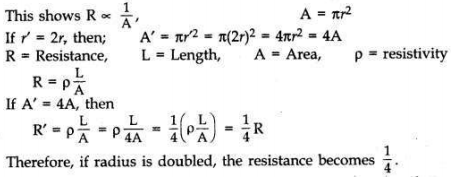
Question 19:
Explain why carbon forms compounds mainly by covalent bond. Explain in brief two main reasons for carbon forming a large number of compounds. Why does carbon form strong bonds with most other elements?
Answer:

Since, carbon has 4 electrons in its outermost shell so, it should either lose or gain 4 electrons to achieve the inert gas configuration and become stable.
(i) It could gain four electrons forming C4- anion. But it would be difficult for the nucleus with six protons to hold on to ten electrons due to inter electronic repulsion.
(ii) It could lose 4 electrons forming C4+ cation. But it would require a large amount of energy to remove four electrons from its outermost shell.
Thus, it forms compounds mainly by covalent bonds.
Two properties of carbon which lead to huge number of carbon compounds are:
(i) Catenation. Catenation is the unique property of carbon atoms to form bonds with other atoms of carbon giving rise to large molecules.
(ii) Tetravalency. Since carbon has a valency of 4, it is capable of bonding with 4 other atoms of carbon or atoms of some other monovalent element.
The reason for the formation of strong bonds by the carbon atoms is their small atomic size. Due to the small size of carbon atoms their nuclei hold the shared pairs of electrons between atoms strongly, leading to the formation of strong covalent bonds. The carbon atoms also form strong covalent bonds with the atoms of other elements such as hydrogen, oxygen, nitrogen, sulphur, chlorine and other elements.
Question 20:
How many pairs of chromosomes are present in human beings? Out of these how many are sex chromosomes? How many types of sex chromosomes are found in human beings? “The sex of a newborn child is a matter of chance and none of the parents may be considered responsible for it”. Draw a flow chart showing determination of sex of a newborn to justify this statement.
Answer:
• There are 23 pairs of chromosomes present in human beings.
• There is 1 pair of sex chromosomes present in human beings.
• The chromosomes which determine the sex of a person are called sex chromosomes. There are two types of sex chromosomes, one is called X chromosome and the other is called Y chromosome. Males contain one X chromosome and one Y chromosome (XY), while females contain two X chromosomes (XX).
Flow chart showing determination of sex of a child.
(i)
A male has one X-chromosome and one Y-chromosome. Thus half the male
gametes have X-chromosomes and the other half have Y-chromosomes.
(ii) A female has two X-chromosomes. Thus all female gametes have only X- chromosomes.
(iii) If a sperm carrying Y-chromosome fertilises an ovum carrying X- chromosome, then the child born will be a boy.
(iv) If a sperm carrying X-chromosome fertilises an ovum carrying X- chromosome, then the child born will be a girl.
(ii) A female has two X-chromosomes. Thus all female gametes have only X- chromosomes.
(iii) If a sperm carrying Y-chromosome fertilises an ovum carrying X- chromosome, then the child born will be a boy.
(iv) If a sperm carrying X-chromosome fertilises an ovum carrying X- chromosome, then the child born will be a girl.

Therefore it is the sperm from the father which determines the sex of the child.
Question 21:
“A convex lens can form a magnified erect as well as magnified inverted image of an object placed in front of it.” Draw ray diagram to justify this statement stating the position of the object with respect to the lens in each case.
An object of height 4 cm is placed at a distance of 20 cm from a concave lens of focal length 10 cm. Use lens formula to determine the position of the image formed. 5 Answer:
Answer:
• A convex lens can form a magnified erect image when the object is placed between the optical centre and principal focus of the convex lens (i.e., between O and F’).

• A convex lens can form a magnified inverted image when the object is placed between focus and the centre of curvature (i.e., between F’ and 2F’).

• Object height, n = 4 cm;
Object distance, u = -20 cm Nature of the lens = concave lens; Focal length = f = -10 cm Image distance, v = ?
According to the lens formula,

So, image is formed at 6.66 cm from lens on same side. Image is erect, virtual and diminished in size.
SECTION B
Question 22:An iron nail is dipped in the solution of copper sulphate for about 30 minutes, state the change in colour observed. Give the reason for the change.
Answer:
Iron is more reactive than Cu. So it displaces Cu from copper sulphate solution. Thus, blue colour of the CuS04 solution fades and colour of the solution turns green due to the formation of ferrous sulphate Solution. This is a displacement reaction.
CuSO4 (aq) + Fe (s) —> FeSO4 (aq) + Cu (s)
(Blue) (Green)
Question 23:
A student while verifying Ohm’s law calculated the value of resistance of the resistor for each set of observation. However, the values of resistance were slightly different from the actual value. Is his experiment wrong? Justify your answer.
Answer:
His experiment is correct since some of the current is utilized to overcome the resistance of the wires of the circuit and instruments like ammeter and
voltmeter. Thus the experimental values of resistance were different from the actual value of resistance.
Question 24:
Draw a labelled diagram of stomatal apparatus with closed stomatal pore.
Answer:

Question 25:
List two observations which you make when you add a pinch of sodium hydrogen carbonate to acetic acid in a test tube. Write the chemical equation for the reaction that occurs.
Answer:
When a pinch of sodium hydrogen carbonate is added to acetic acid in a test tube, there are two observations:
(i) Brisk effervescence
(ii) Evolution of a colourless and odourless gas which is CO2.
CH3 COOH (aq) + NaHCO3 (s) -> CH3 COONa (aq) + H2O (I) + CO2 (g) ↑
Question 26:
Name the type of asexual reproduction in which two individuals are formed from a single parent and the parental identity is lost.
Draw the initial and the final stages of this type of reproduction. State the event with which this reproduction starts.
Answer:
• Binary fission is a type of asexual reproduction in which two individuals are formed from a single parent and the parental identity is lost.
• This reproduction starts with the elongation of the nucleus.

Question 27:
To find the image-distance for varying object-distances in case of a convex
lens, a student obtains on a screen a sharp image of a bright object placed very far from the lens. After that he gradually moves the object towards the lens and each time focuses
(a) In which direction-towards or away from the lens, does he move the screen to focus the object?
(b) What happens to the size of image—does it increase or decrease?
(c) What happen when he moves the object very close to the lens?
Answer:
fa) He moves the screen away from iens to focus the object, l b) The size of the image increases.
Ic) When the object is placed very close to the lens, then no image will be formed on the screen. As now image formed is erect and virtual.
CBSE Class 10th Science Sample Paper 2019 part 4
Board – Central Board of Secondary Education, cbse.nic.in
Subject – CBSE Class 10 Science
Year of Examination – 2019.
II. All questions are compulsory.
III. All questions of Section A and all questions of Section B are to be attempted separately.
IV. Question numbers I to 2 in Section A are one mark questions. These are to be answered in one word or in one sentence.
V. Question numbers 3 to 5 in Section A are two marks questions. These are to be answered in about 30 words each.
VI. Question numbers 6 to 15 in Section A are three marks questions. These are to be answered in about 50 words each.
VII. Question numbers 16 to 21 in Section A are five marks questions. These are to be answered in about 70 words each.
VIII. Question numbers 22 to 27 in Section B are questions based on practical skills and are five marks questions.
Name one plant hormone which inhibits growth. Write its one more function.
Answer:
Abscisic acid is a plant hormone which functions mainly as a growth inhibitor.
• It promotes the dormancy in seeds and buds. It promotes the closing of stomata.
• It promotes the wilting and falling of leaves.
Question 2:
Write the name and structure of an alcohol with three carbon atoms in its molecule.
Answer:
Name of an alcohol: 1-Propanol
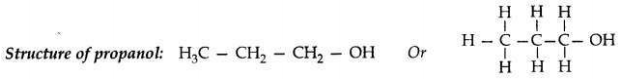
Question 3:
Name the type of mirrors used in the design of solar furnaces. Explain how high temperature is achieved by this device.
Answer:
Concave mirrors are used in the design of solar furnaces. When a solar furnace is placed at the focus of a large concave mirror (called reflector), it focuses a parallel beam of light on the furnace, as a result a temperature is achieved after some time.
Question 4:
“What was Chipko Andolan”? How did this Andolan ultimately benefit the local people and the environment?
Answer:
Chipko Movement. Chipko Movement is an example of contribution of common people towards the conservation of forests. The Chipko Movement also called ’Hug the tree’ movement originated from an incident in a remote village called ’Reni’ in Garhwal (Himalayas), where the people of this village clasped the tree trunks with their arms to protect them from being cut down by a contractor’s workers. The people acted this way because they knew that this mass deforestation would spoil their healthy environment. The forest trees were thus saved.
The Chipko Movement quickly spread across all the communities and helped in the conservation of forests and thus helped in safeguarding the environment.
Question 5:
A metal ’M’ is found in nature as its carbonate. It is used in the galvanization of iron.
Identify ’M’ and name its ore. How will you convert this ore into free metal?
Answer:
’M’ = Zinc metal
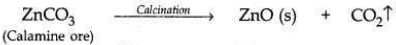
Zinc occurs as Zinc Carbonate in calamine ore, ZnCCh.
Zinc can be extracted from the ore by:
(i) Zinc Carbonate is first converted into Zinc Oxide by calcination. When calamine ore (zinc carbonate) is heated strongly in the absence of air, it decomposes to form zinc oxide and carbon dioxide.
(ii) Zinc metal is then extracted from zinc oxide bv reduction with carbon (coke).
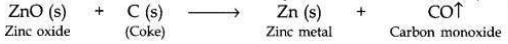
Question 6:
(i) Name two waste products which are stored in old xylem in plants.
(ii) Name the process by which plants get rid of excess water. Name the pores through which this process takes place.
Answer:
(i) Resin and gums are the two wastes which are stored in old xylem in plants.
(ii) • Transpiration is the process by which plants get rid of excess water.
• Stomatal pores are the pores through which transpiration takes place.
Question 7:
(a) In electrolysis of water, why is the volume of gas collected over one electrode double that of gas collected over the other electrode?
(b)
(i) What is observed when a solution of potassium iodide is added to a solution of lead nitrate taken in a test tube?
(ii) What type of reaction is this?
(iii) Write a balanced chemical equation to represent the above reaction.
Answer:
(a) In electrolysis of water (H2O), the hydrogen goes to one test tube and oxygen goes to to another. The two electrodes collect H and O separately.
Since water (Hsub>2O) consists of 2 parts of hydrogen and 1 part of oxygen, so, the volume of hydrogen gas (Hsub>2) collected over cathode (negative electrode) is double the volume of oxygen gas (Osub>2) collected over anode (positive electrode).
(b)
(i) When potassium iodide solution is added to lead nitrate solution, then a yellow precipitate of lead iodide is produced along with potassium nitrate solution.
(ii) This is a double displacement reaction.
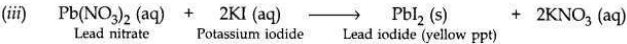
OR
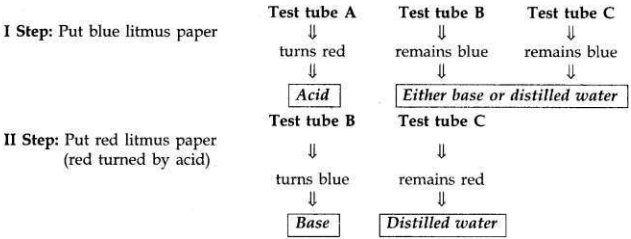
You are provided with three test tubes A, B and C which contain distilled water, acidic solution and basic solution respectively. If you are given blue litmus paper only, how will you identify the contents of each test tube?
Answer:
Question 8:
State the function of receptors in our body. Think of any three situations where receptors in the body do not work properly. Mention the problems which are likely to arise.
Answer:
In the given electric circuit if the current flowing through 3 Ω resistor is 1A, find the voltage of the battery and the current I drawn from it.
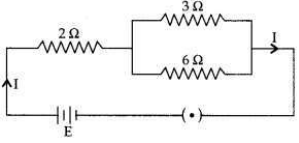
Answer:
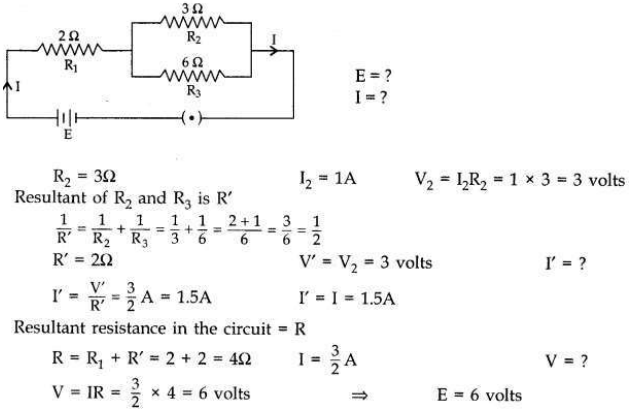
Question 10:
State the principle of working of ocean thermal conversion plant. Explain how the plant works? Write one essential condition for it to operate properly.
Answer:
The energy available due to the difference in the temperature of water at the surface of the ocean and at deeper levels is called Ocean Thermal Energy (OTE).
Condition for it to operate properly:
An aldehyde as well as a ketone can be represented by the same molecular formula, say C3H6O. Write their structures and name them. State the relation between the two in the language of science.
Answer:
Molecular formula: C3H6O

• They are called isomers because both have same molecular formula but different structural formula (having different functional groups). They do not have same physical properties since their functional group is different.
Question 12:
An element ’X’ has mass number 35 and number of neutrons 18. Write atomic number and electronic configuration of ’X’. Also write group number, period number and valency of’X’.
Answer:
’X’ –> Mass number = 35
No. of neutrons = 18

Question 13:
Define reproduction. How does it help in providing stability to the population of species?
Answer:
Explain the term “Regeneration” as used in relation to reproduction of organisms. Describe briefly how regeneration is carried out in multicellular organisms like Hydra.
Answer:
“Two areas of study namely ’evolution’ and ’classification’ are interlinked”. Justify this statement.
Answer:
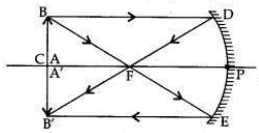
The image of an object formed by a mirror is real, inverted and is of magnification -1. If the image is at a distance of 40 cm from the mirror, where is the object placed? Where would the image be if the object is moved 20 cm towards the mirror? State reason and also draw ray diagram for the new position of the object to justify your answer.
Answer:
Magnification, m = -1; Image is real and inverted; Image distance = -40 cm
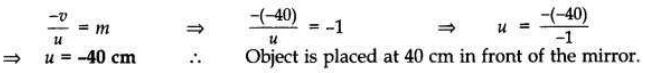
When object distance is equal to the image distance and image is real, then the object is placed at C. If the object is moved 20 cm towards the mirror, then its new position would be at the focus of the mirror.
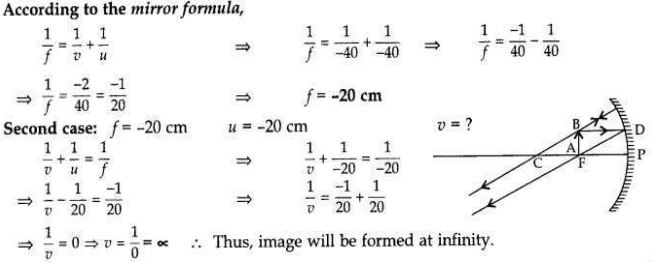
Question 16:
(a) Explain any two physical properties of ionic compounds giving reason.
(b) List any two metals found in free state in earth’s crust. Where are they located in activity series?
(c) Metals towards the top of the activity series can not be obtained from their compounds by reducing with carbon. Why?
Answer:
(a) Physical properties of ionic compounds:
(c) Metals towards the top of the activity series are highly reactive. The oxides of highly reactive metals are very stable and can not be reduced by ’carbon’ to obtain free metals because these metals have more affinity for oxygen than carbon.
Question 17:
(a) Define reflex action. State its significance.
(b) How do plants respond to external stimuli?
Answer:
(a) A reflex action is an automatic response to a stimulus. The simplest form of response in the nervous system is reflex action. This is a rapid, automatic response to a stimulus which is not under the voluntary control of the brain. It is described as an involuntary action. The pathway taken by nerve impulses in a reflex action is called the reflex arc. Reflex actions are the actions which we perform without thinking to protect ourselves. For example, coughing is a reflex action which clears our windpipe. The pupils of our eyes get smaller in bright light. This reflex action protects the retina of our eyes from damage due to much light. The pupils of our eyes get bigger in dim light so as to help us see properly even in dim light.
(b) Plants respond to external stimuli such as light, touch, etc. A growth movement of a plant part in response to an external stimulus in which the direction of stimuli determines the direction of response in called tropism.
State Ohm’s law. Draw a labelled circuit diagram to verify this law in the laboratory. If you draw a graph between the potential difference and current flowing through a metallic conductor, what kind of curve will you get? Explain how would you use this graph to determine the resistance of the conductor.
Answer:
Ohm’s law states that the electric current, through a conductor, is directly proportional to the potential difference across its two ends when, other physical conditions like temperature, etc., remain constant.
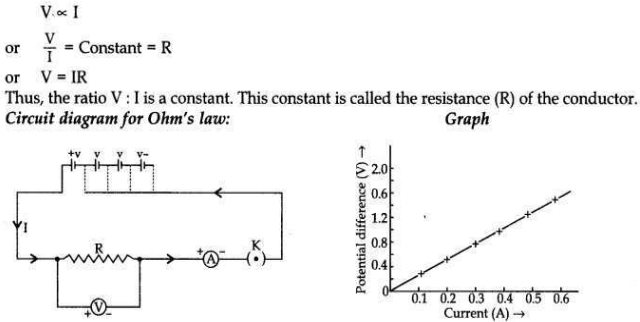
Explanation. If a graph is drawn between the potential difference (V) and current (I), the graph is found to be a straight line passing through the origin. This shows current is directly proportional to the potential difference. Thus the ratio V/l remains constant. This constant is called the resistance of the conductor. The gradient of the straight line graph is related to the resistance (R) of the conductor.
OR
(a) A coil of insulated wire is connected to a galvanometer. What would be seen if a bar magnet with its south pole towards one face of the coil is
(b) Name and define the phenomenon involved in above activities.
(c) Name the rule which can determine the direction of current in each case.
Answer:
(a) A coil of insulated wire is connected to a galvanometer and if a bar magnet with its south pole towards one face of the coil is
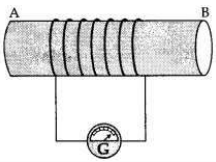
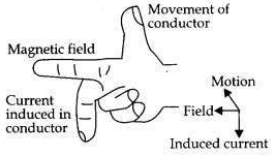
(c) The direction of induced current is determined by ’Fleming’s Right Hand Rule’.
Question 19:
A carbon compound ’P’ on heating with excess cone. H2SO4 forms another carbon compound ’Q’ which on addition of hydrogen in the presence of nickel catalyst forms a saturated carbon compound ’R’. One molecule of ’R’ on combustion, forms two molecules of carbon dioxide and three molecules of water. Identify P, Q and R and write chemical equations for the reactions involved.
Answer:
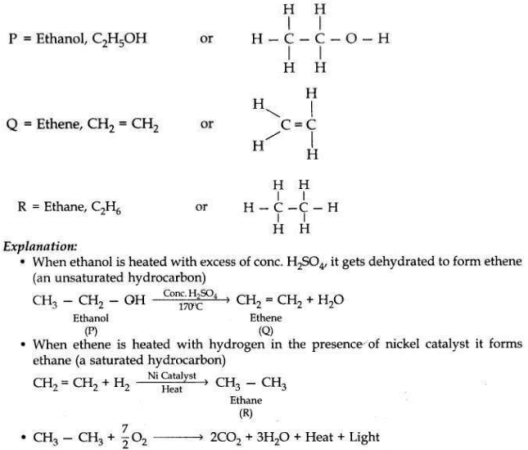
Question 20:
Define evolution. How does it occur? Describe how fossils provide us evidences in support of evolution.
Answer:
Evolution. Evolution is the sequence of gradual changes which take place in the primitive organisms over millions of years in which new species are produced.
How it occurs?
It is desired to obtain an erect image of an object, using concave mirror of focal length of 12 cm.
(i) What should be the range of distance of an object placed in front of the mirror?
(ii) Will the image be smaller or larger than the object? Draw a ray diagram to show the formation of image in this case.
(iii) Where will the image of this object be, if it is placed 24 cm in front of the mirror? Draw a ray diagram for this situation also to justify your answer.
Show the positions of the pole, the principal focus and the centre of curvature in the above ray diagrams.
Answer:
In a concave mirror an erect image will be obtained when the object is placed between
pole and focus of the mirror.
Focal length,/= 12 cm
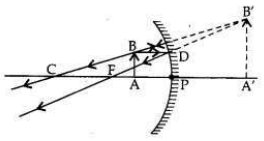
(i) Therefore, the range of object distance is between 0 cm to <12 cm (from zero to less than 12 cm).
(ii) Image formed will be magnified, i.e., larger than the object.
(iii) If the object is placed at 24 cm in front of the mirror, it means that object is placed at 2 fi.e., at the centre of curvature (at C) of the mirror.
The real, inverted and same size (of the object) image will also be formed at 24 cm.
To study the dependence of potential difference (V) on current I across Resistor (R), four circuit diagrams are prepared.
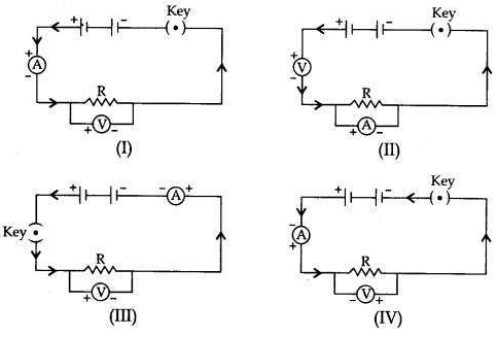
(i) Select the circuit diagrams which are correct.
(ii) Give reason for the circuit diagrams which are not correct.
Answer:
(i) Circuit diagrams (I) and (III) are correct.
(ii) Circuit diagram (II) is incorrect because ammeter is always connected in series with the resistor and voltmeter is always connected in parallel to the resistor.
Circuit diagram (IV) is incorrect because the negative (-ve) terminal of the ammeter and the voltmeter is connected to the positive (+ve) terminal of the battery and there is positive (+ve) terminals connection.
Question 23:
(i) While studying the combination reaction on adding water to quick lime, name the product formed and write its colour.
(ii) While studying the decomposition reaction by heating ferrous sulphate crystals in a test-tube, a product is formed in the test-tube. Name the product and write its colour.
Answer:
(i) Quicklime (CaO) reacts vigorously with water to form slaked lime [Ca(OH)2] which is white in colour.

(ii) When ferrous sulphate is heated strongly, it decomposes to form brown coloured ferric oxide and sulphur dioxide gas and sulphur trioxide gas.

Question 24:
Identify the observed various parts of temporary mount of well stained leaf peel, when focussed under the high power of a microscope.
Answer:
Parts of temporary mount of well stained leaf peel:
(i) Stomatal aperture (opened)/(closed)
(ii) Guard cells
(iii) Chloroplast
(iv) Nucleus
(v) Epidermal cells
Question 25:
A student adds a spoon full of powdered sodium hydrogen carbonate to a flask containing ethanoic acid. List two main observations, he must note in his note book, about the reaction that takes place. Also write chemical equation for the reaction.
Answer:
When sodium hydrogen carbonate is added to a flask containing ethanoic acid, then
Question 26:
A student is observing a permanent slide showing sequentially the different stages of asexual reproduction taking place in yeast. Name this process and draw diagrams of what he observes in a proper sequence.
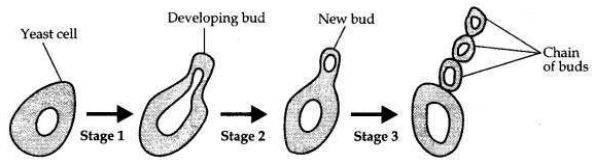
Answer:
The process of reproduction in yeast is budding.
Question 27:
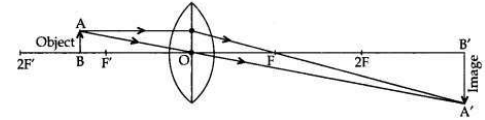
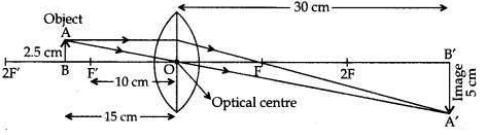
An object of height 2.5 cm is placed at a distance of 15 cm from the optical centre ’O’ of a convex lens of focal length 10 cm. Draw a ray diagram to find the position and size of the image formed. Mark optical centre ’O’, principal focus F and height of the image on the diagram.
Answer:
We hope the Solved CBSE Sample Papers for Class 10 Science Set 4, help you. If you have any query regarding CBSE Sample Papers for Class 10 Science Solved Set 4, drop a comment below and we will get back to you at the earliest.
Subject – CBSE Class 10 Science
Year of Examination – 2019.
Solved CBSE Sample Papers for Class 10 Science Set 4
GENERAL INSTRUCTIONS
I. The Question Paper comprises of two sections, A and B. You are to attempt both the sections.II. All questions are compulsory.
III. All questions of Section A and all questions of Section B are to be attempted separately.
IV. Question numbers I to 2 in Section A are one mark questions. These are to be answered in one word or in one sentence.
V. Question numbers 3 to 5 in Section A are two marks questions. These are to be answered in about 30 words each.
VI. Question numbers 6 to 15 in Section A are three marks questions. These are to be answered in about 50 words each.
VII. Question numbers 16 to 21 in Section A are five marks questions. These are to be answered in about 70 words each.
VIII. Question numbers 22 to 27 in Section B are questions based on practical skills and are five marks questions.
SECTION A
Question 1:Name one plant hormone which inhibits growth. Write its one more function.
Answer:
Abscisic acid is a plant hormone which functions mainly as a growth inhibitor.
• It promotes the dormancy in seeds and buds. It promotes the closing of stomata.
• It promotes the wilting and falling of leaves.
Question 2:
Write the name and structure of an alcohol with three carbon atoms in its molecule.
Answer:
Name of an alcohol: 1-Propanol
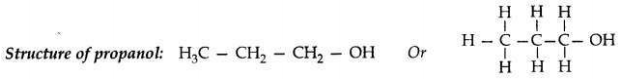
Question 3:
Name the type of mirrors used in the design of solar furnaces. Explain how high temperature is achieved by this device.
Answer:
Concave mirrors are used in the design of solar furnaces. When a solar furnace is placed at the focus of a large concave mirror (called reflector), it focuses a parallel beam of light on the furnace, as a result a temperature is achieved after some time.
Question 4:
“What was Chipko Andolan”? How did this Andolan ultimately benefit the local people and the environment?
Answer:
Chipko Movement. Chipko Movement is an example of contribution of common people towards the conservation of forests. The Chipko Movement also called ’Hug the tree’ movement originated from an incident in a remote village called ’Reni’ in Garhwal (Himalayas), where the people of this village clasped the tree trunks with their arms to protect them from being cut down by a contractor’s workers. The people acted this way because they knew that this mass deforestation would spoil their healthy environment. The forest trees were thus saved.
The Chipko Movement quickly spread across all the communities and helped in the conservation of forests and thus helped in safeguarding the environment.
Question 5:
A metal ’M’ is found in nature as its carbonate. It is used in the galvanization of iron.
Identify ’M’ and name its ore. How will you convert this ore into free metal?
Answer:
’M’ = Zinc metal
Zinc occurs as Zinc Carbonate in calamine ore, ZnCCh.
Zinc can be extracted from the ore by:
(i) Zinc Carbonate is first converted into Zinc Oxide by calcination. When calamine ore (zinc carbonate) is heated strongly in the absence of air, it decomposes to form zinc oxide and carbon dioxide.
(ii) Zinc metal is then extracted from zinc oxide bv reduction with carbon (coke).
Question 6:
(i) Name two waste products which are stored in old xylem in plants.
(ii) Name the process by which plants get rid of excess water. Name the pores through which this process takes place.
Answer:
(i) Resin and gums are the two wastes which are stored in old xylem in plants.
(ii) • Transpiration is the process by which plants get rid of excess water.
• Stomatal pores are the pores through which transpiration takes place.
Question 7:
(a) In electrolysis of water, why is the volume of gas collected over one electrode double that of gas collected over the other electrode?
(b)
(i) What is observed when a solution of potassium iodide is added to a solution of lead nitrate taken in a test tube?
(ii) What type of reaction is this?
(iii) Write a balanced chemical equation to represent the above reaction.
Answer:
(a) In electrolysis of water (H2O), the hydrogen goes to one test tube and oxygen goes to to another. The two electrodes collect H and O separately.
Since water (Hsub>2O) consists of 2 parts of hydrogen and 1 part of oxygen, so, the volume of hydrogen gas (Hsub>2) collected over cathode (negative electrode) is double the volume of oxygen gas (Osub>2) collected over anode (positive electrode).
(b)
(i) When potassium iodide solution is added to lead nitrate solution, then a yellow precipitate of lead iodide is produced along with potassium nitrate solution.
(ii) This is a double displacement reaction.
OR
You are provided with three test tubes A, B and C which contain distilled water, acidic solution and basic solution respectively. If you are given blue litmus paper only, how will you identify the contents of each test tube?
Answer:
- First take three strips of blue litmus paper and dip one in each test tube.
- The liquid in test tube A turns blue litmus to red. It means test tube A contains acid because (acid turns blue litmus to red).
- If other two test tubes do not change the colour of blue litmus paper, it shows that one of them contains a base and the other contains distilled water.
- Now put the blue litmus paper which is turned red by the acid of test tube A in the remaining two test tubes.
- If the liquid of test tube B turns that red litmus paper to blue again, it shows that it is a base.
- The liquid in test tube C does not turn the colour of either blue litmus or red litmus thus is distilled water.

Question 8:
State the function of receptors in our body. Think of any three situations where receptors in the body do not work properly. Mention the problems which are likely to arise.
Answer:
- Receptors are present in all parts of our body, for example, in the skin, eyes, nose, tongue, etc.
- A receptor is a cell (or a group of cells) in a sense organ which is sensitive to a particular type of stimulus such as light, sound, smell, taste, heat, pressure, etc.
- The different sense organs contain receptors for detecting different stimuli. The eyes have light receptors called photoreceptors (which can detect light). Ears have sound receptors called photoreceptors (which can detect the sounds).
- The nose has smell receptors called olfactory receptors which can detect the smell. Tongue has taste receptors which are called gustatory receptors which detect taste.
- Skin has receptors called thermoreceptors which detect touch, pressure.
- When receptors do not work properly, the environmental stimuli are not able to create nerve impluses and the body does not respond.
- When receptors are damaged, the external stimuli transferring signals to the brain are not felt.
For example, - during fever, taste buds do not work properly and as a result, taste of the food eaten is not felt properly thus enzyme secretion is also affected.
- when a person is suffering from a cold, the nostrils are filled with mucus. Then smell of the surrounding is not felt properly.
- This is due to interruption in reacting to the sense of smell by the olfactory receptor.
- when skin receptors are damaged, and we accidentally touch a hot object, then our hands might get burnt as the damaged receptor cannot perceive the external stimuli of heat and pain.
In the given electric circuit if the current flowing through 3 Ω resistor is 1A, find the voltage of the battery and the current I drawn from it.

Answer:

Question 10:
State the principle of working of ocean thermal conversion plant. Explain how the plant works? Write one essential condition for it to operate properly.
Answer:
The energy available due to the difference in the temperature of water at the surface of the ocean and at deeper levels is called Ocean Thermal Energy (OTE).
Condition for it to operate properly:
- Ocean thermal energy plants can operate if the temperature difference between the water at the surface and water at depths up to 2 km is 293 K (20°C) or more.
- Working. The devices used to harness ocean thermal energy are called ocean thermal energy conversion power plants (or OTEC power plants).
- A temperature difference of 20°C (or more) between the surface water of ocean and deeper water is needed for operating OTEC power plants.
- In one type of OTEC power plant, the warm surface water of ocean is used to boil a volatile liquid like ammonia or a CFC.
- The high pressure vapours of the liquid are then used to turn the turbine of a generator and produce electricity.
- The colder water from the deeper ocean is pumped up to cool the used up vapours and convert them again into a liquid. This process is repeated again and again.
An aldehyde as well as a ketone can be represented by the same molecular formula, say C3H6O. Write their structures and name them. State the relation between the two in the language of science.
Answer:
Molecular formula: C3H6O

• They are called isomers because both have same molecular formula but different structural formula (having different functional groups). They do not have same physical properties since their functional group is different.
Question 12:
An element ’X’ has mass number 35 and number of neutrons 18. Write atomic number and electronic configuration of ’X’. Also write group number, period number and valency of’X’.
Answer:
’X’ –> Mass number = 35
No. of neutrons = 18

Question 13:
Define reproduction. How does it help in providing stability to the population of species?
Answer:
- The production of new organisms from the existing organisms of the same species is known as reproduction. It is essential for the survival of a species on earth.
- It helps in replacing the lost section of the population due to death and various other causes.
- Populations of organisms live in well defined places called niches in the ecosystem using their ability to reproduce.
- Reproduction involves DNA copying which is the source of information for making proteins thereby controlling body design.
- These body designs allow the organism to use a particular niche for the stability of the population of a species.
- Minor variations may also lead to the stability of the species.
Explain the term “Regeneration” as used in relation to reproduction of organisms. Describe briefly how regeneration is carried out in multicellular organisms like Hydra.
Answer:
- Regeneration is a mode of asexual reproduction in some organisms. The process of getting back a full organism from its body parts is called regeneration.
- The simple multicellular animals like hydra and planaria show regeneration.
- If the body of hydra gets cut into a number of pieces, then each body piece can regenerate into a complete hydra by growing all the missing parts.
- The regeneration of an organism from its cut body part occurs by the process of growth and development.
- The cells of cut body parts divide rapidly to make a ball of cells.
- The cells then become specialised to form different types of tissues which again form various organs and body parts.
“Two areas of study namely ’evolution’ and ’classification’ are interlinked”. Justify this statement.
Answer:
- Classification of organisms is based on relative similarities and differences in their internal and external structures.
- Similarities among organisms will allow us to group them and study the groups and classify them. Some basic characteristics will be shared by most organisms.
- The more characteristics the two species will have in common, the more closely they are related. The more closely they are related, the more recently they have had a common ancestor.
- So we can say that classification of a species is in fact a reflection of their evolutionary relationship.
The image of an object formed by a mirror is real, inverted and is of magnification -1. If the image is at a distance of 40 cm from the mirror, where is the object placed? Where would the image be if the object is moved 20 cm towards the mirror? State reason and also draw ray diagram for the new position of the object to justify your answer.
Answer:
Magnification, m = -1; Image is real and inverted; Image distance = -40 cm
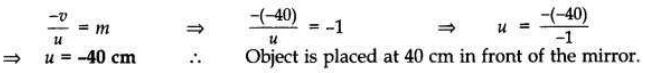
When object distance is equal to the image distance and image is real, then the object is placed at C. If the object is moved 20 cm towards the mirror, then its new position would be at the focus of the mirror.

Question 16:
(a) Explain any two physical properties of ionic compounds giving reason.
(b) List any two metals found in free state in earth’s crust. Where are they located in activity series?
(c) Metals towards the top of the activity series can not be obtained from their compounds by reducing with carbon. Why?
Answer:
(a) Physical properties of ionic compounds:
(i)
Ionic compounds are usually crystalline solids because their oppositely
charged ions attract one another strongly and form a regular crystal
structure.
(ii) Ionic compounds have high melting and high boiling points. The ionic compounds are made up of +ve and -ve ions. There is a strong force of attraction between the oppositely charged ions, so a lot of heat energy is required to break this force of attraction and melt or boil the ionic compounds.
(b) Gold and platinum metals are
found in free state in the earth’s crust. These metals are located at
the bottom in the activity series.(ii) Ionic compounds have high melting and high boiling points. The ionic compounds are made up of +ve and -ve ions. There is a strong force of attraction between the oppositely charged ions, so a lot of heat energy is required to break this force of attraction and melt or boil the ionic compounds.
(c) Metals towards the top of the activity series are highly reactive. The oxides of highly reactive metals are very stable and can not be reduced by ’carbon’ to obtain free metals because these metals have more affinity for oxygen than carbon.
Question 17:
(a) Define reflex action. State its significance.
(b) How do plants respond to external stimuli?
Answer:
(a) A reflex action is an automatic response to a stimulus. The simplest form of response in the nervous system is reflex action. This is a rapid, automatic response to a stimulus which is not under the voluntary control of the brain. It is described as an involuntary action. The pathway taken by nerve impulses in a reflex action is called the reflex arc. Reflex actions are the actions which we perform without thinking to protect ourselves. For example, coughing is a reflex action which clears our windpipe. The pupils of our eyes get smaller in bright light. This reflex action protects the retina of our eyes from damage due to much light. The pupils of our eyes get bigger in dim light so as to help us see properly even in dim light.
(b) Plants respond to external stimuli such as light, touch, etc. A growth movement of a plant part in response to an external stimulus in which the direction of stimuli determines the direction of response in called tropism.
- If the growth of a plant part is towards the stimuli, it is called positive tropism.
- If the growth of a plant part is away from the stimulus, then it is called negative tropism.
- The movement of a plant part in response to light is called phototropism.
- The movement of a plant part in response to gravity is called geotropism.
- The movement of a plant part in response to chemicals is called chemotropism.
- The movement of a plant part in response to water is called hydrotropism.
- The directional growth movement of a plant part in response to the touch of an object is called thigmotropism.
- Nasties (or Nastic Movements). The movement of a plant part in response to an external stimulus in which the direction of response is not determined by the direction of stimulus is called nastic movement.
- The folding up of a the leaves of a sensitive plant on touching is an example of nastic movement. Here the stimulus is touch.
- The opening up of the petals of dandelion flowers in morning in bright light and closing in the evening when the light fades is an example of nastic movement. In this case the stimulus is light.
State Ohm’s law. Draw a labelled circuit diagram to verify this law in the laboratory. If you draw a graph between the potential difference and current flowing through a metallic conductor, what kind of curve will you get? Explain how would you use this graph to determine the resistance of the conductor.
Answer:
Ohm’s law states that the electric current, through a conductor, is directly proportional to the potential difference across its two ends when, other physical conditions like temperature, etc., remain constant.

Explanation. If a graph is drawn between the potential difference (V) and current (I), the graph is found to be a straight line passing through the origin. This shows current is directly proportional to the potential difference. Thus the ratio V/l remains constant. This constant is called the resistance of the conductor. The gradient of the straight line graph is related to the resistance (R) of the conductor.
OR
(a) A coil of insulated wire is connected to a galvanometer. What would be seen if a bar magnet with its south pole towards one face of the coil is
(i) moved quickly toward it
(ii) moved quickly away from it
(iii) placed near its one face?
These activities are then repeated with north pole of the magnet. What will be the observations?(ii) moved quickly away from it
(iii) placed near its one face?
(b) Name and define the phenomenon involved in above activities.
(c) Name the rule which can determine the direction of current in each case.
Answer:
(a) A coil of insulated wire is connected to a galvanometer and if a bar magnet with its south pole towards one face of the coil is
(i) moved quickly towards it, the galvanometer is deflected towards the left.
(ii) moved quickly away from it, the galvanometer is deflected towards the right.
(iii) placed near its one face, the deflection of the galvanometer is zero.
If this activity is repeated with north pole of the magnet(ii) moved quickly away from it, the galvanometer is deflected towards the right.
(iii) placed near its one face, the deflection of the galvanometer is zero.

(i) If the magnet is pushed into the coil, the A- galvanometer is deflected towards the right.
(ii) It the magnet is withdrawn from the coil, the galvanometer is deflected towards the left.
(iii) If the magnet is held stationary inside the coil, the deflection of the galvanometer is zero.
(b)
This phenomenon involved in this activity is ’Electromagnetic
Induction’. The production of electric current by moving a magnet inside
a fixed coil of wire is called electromagnetic induction.(ii) It the magnet is withdrawn from the coil, the galvanometer is deflected towards the left.
(iii) If the magnet is held stationary inside the coil, the deflection of the galvanometer is zero.

(c) The direction of induced current is determined by ’Fleming’s Right Hand Rule’.
Question 19:
A carbon compound ’P’ on heating with excess cone. H2SO4 forms another carbon compound ’Q’ which on addition of hydrogen in the presence of nickel catalyst forms a saturated carbon compound ’R’. One molecule of ’R’ on combustion, forms two molecules of carbon dioxide and three molecules of water. Identify P, Q and R and write chemical equations for the reactions involved.
Answer:

Question 20:
Define evolution. How does it occur? Describe how fossils provide us evidences in support of evolution.
Answer:
Evolution. Evolution is the sequence of gradual changes which take place in the primitive organisms over millions of years in which new species are produced.
How it occurs?
- It is through the constant process of evolution taking place in the organisms since the origin of life that such an enormous variety of plants and animals have come to exist on this earth at present.
- There is an inbuilt tendency to variation during reproduction due to errors in DNA copying and as a result of sexual reproduction.
- Fossils are the remains of impressions of dead plants or animals which died millions of years ago. The study of fossils helps us to know about the evolution of species.
- Fossils tell us how new species are developed from the old.
- Fossilsprovide evidence of evolution by revealing characteristics of past organisms and the changes that have occured in these organisms to give rise to present organisms.
- Therefore, fossils have an importance in deciding evolutionary relationship.
- For example, a fossil called Archaeopteryx has feathered wings like birds but teeth and tail like reptiles hence suggesting that birds and reptiles had a common ancestor.
It is desired to obtain an erect image of an object, using concave mirror of focal length of 12 cm.
(i) What should be the range of distance of an object placed in front of the mirror?
(ii) Will the image be smaller or larger than the object? Draw a ray diagram to show the formation of image in this case.
(iii) Where will the image of this object be, if it is placed 24 cm in front of the mirror? Draw a ray diagram for this situation also to justify your answer.
Show the positions of the pole, the principal focus and the centre of curvature in the above ray diagrams.
Answer:
In a concave mirror an erect image will be obtained when the object is placed between
pole and focus of the mirror.
Focal length,/= 12 cm

(i) Therefore, the range of object distance is between 0 cm to <12 cm (from zero to less than 12 cm).
(ii) Image formed will be magnified, i.e., larger than the object.
(iii) If the object is placed at 24 cm in front of the mirror, it means that object is placed at 2 fi.e., at the centre of curvature (at C) of the mirror.
The real, inverted and same size (of the object) image will also be formed at 24 cm.

SECTION B
Question 22:To study the dependence of potential difference (V) on current I across Resistor (R), four circuit diagrams are prepared.

(i) Select the circuit diagrams which are correct.
(ii) Give reason for the circuit diagrams which are not correct.
Answer:
(i) Circuit diagrams (I) and (III) are correct.
(ii) Circuit diagram (II) is incorrect because ammeter is always connected in series with the resistor and voltmeter is always connected in parallel to the resistor.
Circuit diagram (IV) is incorrect because the negative (-ve) terminal of the ammeter and the voltmeter is connected to the positive (+ve) terminal of the battery and there is positive (+ve) terminals connection.
Question 23:
(i) While studying the combination reaction on adding water to quick lime, name the product formed and write its colour.
(ii) While studying the decomposition reaction by heating ferrous sulphate crystals in a test-tube, a product is formed in the test-tube. Name the product and write its colour.
Answer:
(i) Quicklime (CaO) reacts vigorously with water to form slaked lime [Ca(OH)2] which is white in colour.

(ii) When ferrous sulphate is heated strongly, it decomposes to form brown coloured ferric oxide and sulphur dioxide gas and sulphur trioxide gas.

Question 24:
Identify the observed various parts of temporary mount of well stained leaf peel, when focussed under the high power of a microscope.
Answer:
Parts of temporary mount of well stained leaf peel:
(i) Stomatal aperture (opened)/(closed)
(ii) Guard cells
(iii) Chloroplast
(iv) Nucleus
(v) Epidermal cells
Question 25:
A student adds a spoon full of powdered sodium hydrogen carbonate to a flask containing ethanoic acid. List two main observations, he must note in his note book, about the reaction that takes place. Also write chemical equation for the reaction.
Answer:
When sodium hydrogen carbonate is added to a flask containing ethanoic acid, then
- brisk effervescence will be formed because of CO2 gas escaping from the reaction mixture.
- evolution of colourless and odourless gas. Some amount of heat is evolved during the reaction.
Question 26:
A student is observing a permanent slide showing sequentially the different stages of asexual reproduction taking place in yeast. Name this process and draw diagrams of what he observes in a proper sequence.

Answer:
The process of reproduction in yeast is budding.
Question 27:
An object of height 2.5 cm is placed at a distance of 15 cm from the optical centre ’O’ of a convex lens of focal length 10 cm. Draw a ray diagram to find the position and size of the image formed. Mark optical centre ’O’, principal focus F and height of the image on the diagram.
Answer:

We hope the Solved CBSE Sample Papers for Class 10 Science Set 4, help you. If you have any query regarding CBSE Sample Papers for Class 10 Science Solved Set 4, drop a comment below and we will get back to you at the earliest.
CBSE Class 10th Science Sample Paper 2019
SECTION A
Question 1:
Define photosynthesis.
Answer:
The process by which green plants make their own food (like glucose) from carbon dioxide and water by using solar energy in the presence of chlorophyll is called photosynthesis.
Question 2:Define photosynthesis.
Answer:
The process by which green plants make their own food (like glucose) from carbon dioxide and water by using solar energy in the presence of chlorophyll is called photosynthesis.
List two natural ecosystems.
Answer:
Two natural ecosystems are: Lake and Forest.
Download Class 10th Science Samplel Paper 2019 part 2
SECTION A
Question 1:How is the concentration of hydronium (H3O+) ions affected when a solution of
an acid is diluted?
Answer:
When an acid solution is diluted with water then concentration of (H3O+) ions gets decreased.
Question 2:
In the following food chain, 100 J of energy is available to the lion. How much energy was available to the producer?
Plants —> Deer —> Lion
Answer:
10,00,000 Joules.
Class 10 CBSE Science Sample Paper 2019 Part 1
Solve Sample paper of Class 10th Science 2019
SECTION A
Question 1:
Write the balanced chemical equation with the state symbols of the following reaction: Solutions of Barium chloride and Sodium sulphate in water react to give insoluble Barium sulphate and the solution of Sodium chloride. 1
Answer:
BaCl2(aq) + Na2SO4(aq) → BaSO4(s) + 2 NaCl (aq)
SECTION A
Question 1:
Write the balanced chemical equation with the state symbols of the following reaction: Solutions of Barium chloride and Sodium sulphate in water react to give insoluble Barium sulphate and the solution of Sodium chloride. 1
Answer:
BaCl2(aq) + Na2SO4(aq) → BaSO4(s) + 2 NaCl (aq)
Download Class 9 Sample Papers for 2019
Download Class 9 sample papers for 2019
Board – Central Board of Secondary Education, cbse.nic.in
Subject – CBSE Class 9 Science
Year of Examination – 2019.
Board – Central Board of Secondary Education, cbse.nic.in
Subject – CBSE Class 9 Science
Year of Examination – 2019.
Class 9 Sample Paper Science 2019
SECTION – A
Question 1:What do you mean by the word “mole” ?
Answer:
The mole is the amount of substance that contains the same number of particles (atoms/ions/ molecules/formula units, etc.), as there are atoms in exactly 12 g of carbon-12.
Question 2:
What is the ratio of forces between two masses kept at a certain distance in air to the force between them when kept in any other medium ?
Answer:
1 : 1, because the gravitational force is independent of the nature of the medium in which the bodies are placed.
Question 3:
How do bats move about freely even in dark nights and search out prey ? Name the sound waves used by them and write their frequency.
Answer:
Ultrasonic waves emitted by bat are reflected from prey and received by their ears. So they locate obstacles..
Sound wave – Ultrasound, Frequency – Above 20000 Hz.
Question 4:
What is epidermis? List three functions of epidermis.
Answer:
Epidermis is a single layer of cells that covers all the parts of the plant externally
Functions:
(i) Protection: It protects against loss of water, mechanical injury and invasion by parasitic fungi.
(ii) Secretion: Epidermal cells on the aerial parts of the plant often secrete a waxy layer which further helps in protection from mechanical injury and water loss.
(iii) Absorption: Root hairs present on epidermis of root help ip absorption of water and minerals from the soil.
Question 5:
Write the chemical formulae for the following compounds and find the ratio by mass of the combining elements in each one of them. (N = 14 u, H = 1 u, C = 12 u, O = 16 u, S = 32 u)
(a) Methane
(b) Carbon dioxide
(c) Hydrogen sulphide
Answer:

Question 6:
(a) Do isobars belong to the same element ? Justify your answer by giving one example.
(b) Name two elements whose isotopes are used in the field of medicine.
OR
(a) Mention the steps taken by you or your family members when you fall sick ?
(b) List two ways to treat an infectious disease.
Answer:
(a) No.
(b) (i) An isotope of cobalt (CO – 60) is used in the treatment of cancer.
(ii) An isotope of iodine (1 – 131) is used in the treatment of goitre.
OR
(a)
(i) Ensuring proper balanced diet.
(ii) Maintaining personal and public hygiene.
(iii) Isolation from other member if disease is contagious.
(iv) Proper bed rest and avoiding all type of mental stress.
(v) Maintaining healthy surroundings full of congenial (happy) atmosphere.
(vi) Providing vaccination to prevent further damage and supplements of vitamins and minerals to build immunity against future attack.
(b)
(i) To kill the cause of disease by using antibiotics which can kill the disease causing microbes.
(ii) To reduce the effects of the disease by giving treatment which can reduce the symptoms.
e.g., we can take medicines that bring down fever, reduce pain or loose motions.
Question 7:
Complete the following table :
Atomic number
| Mass number | No. neutron | No. of protons | No. of electrons |
Name of the atomic species
|
–
| 24 | 12 | 12 | – |
–
|
| 17 | – | 18 | – | – |
Chlorine
|
Atomic number
| Mass number | No. of neutron | No.of protons | No. of electrons |
Name of the atomic species
|
12
| 24 | 12 | 12 | 12 | Magnesium |
| 17 | 35 | 18 | 17 | 17 |
Chlorine
|
Explain the following terms in brief:
(a) Bilaterally symmetrical
(b) Triploblastic
(c) Coelom
OR
Differentiate between the following (any one point):
(a) Diploblastic and Triploblastic
(b) Monocots and Dicots
(c) Warm blooded and Cold blooded animals Answer:
(a) It means that, the left and the right halves of the body have the same design.
(b) The animals whose body tissues are derived from three layers of cells are called triploblastic.
(c) The true, internal body cavity in which well developed body organs can be accomodated is called coelom.
OR
(a)
Diploblastic
|
Triploblastic
|
The body is made of two layers of cells (ectoderm and endoderm).
e.g., Hydra, Jelly fish and Sea anemone.
|
The body is made of three layers of cells (ectoderm, endoderm and mesoderm).
e.g., Platyhelminthes, Nematoda and all higher phylums.
|
Monocots
|
Dicots
|
(i)They have only one cotyledon in the seeds,
|
(i) They have two cotyledons in the seeds,
|
(ii) The flower of monocots is (rimerous (multiple of three) and leaf shows parallel venation.
e.gv Grass, wheat, maize, rice, etc
|
(ii) The flower of dicots in pentamerous (multiple of 5) and leaf shows reticulate venation.
e.g., Pea, pulses, mustard, etc.
|
Warm blooded animals
|
Cold blooded animals
|
(i) Their body temperature remains constant irrespective of their surroundings.
|
(i) Their body temperature changes according to the temperature of the surroundings.
|
(ii) They are also known as homeothermals and have energy requirements.
e.gv Aves and mammals. |
(ii) They are also known as poikilothermals and have low energy requirements,
e.g., Pisces, amphibians and reptiles.
|
Area of bottom of a boat is 6 m2 it is floating on water. The boat sinks by is 1 cm, when a man gets on it. What is the mass of the man ?
Answer:
A = 6 cm2
Floating of water
Weight of boat = Buoyant force
mg = vdg; m = vd = 0.06 × 1000 = 60 kg [ v = A × Depth = 6 × 0.01 – 0.06 m3]
Question 10:
(a) A horse exerts a pull on a cart of 300 N. This makes the horse – cart system move with a uniform speed of 18 km h’1 on a level road. Calculate the power developed by the horse in watt.
(b) Prove that the total energy at any point in the path of a dropped mass is ’mg’ times the height from where it is dropped.
Answer:

Question 11:
(a) What do you mean by a pure substance ? Give an example.
(b) Name the appropriate methods to separate the following :
(i) Nitrogen from air
(ii) Dye from blue ink
(iii) Butter cream from milk
(iv) Ammonium chloride from common salt.
Answer:
(a) Pure substance : A pure substance contains only one kind of atoms or molecules and is homogeneous throughout its mass. A pure substance has a fixed composition as well as a fixed melting and boiling point, e.g., iron, water, etc.
(b)
(i) Fractional distillation
(ii) Chromatography
(iii) Centrifugation
(iv) Sublimation
Question 12:
Complete the following flow diagram:
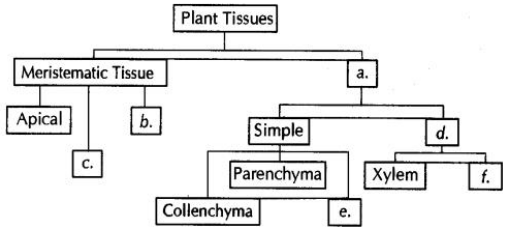
Answer:
(a) Permanent tissue
(b) Lateral meristem
(c) Intercalary meristem
(d) Complex permanent tissues
(e) Sclerenchyma
(f) Phloem
Question 13:
Derive following equations for a uniformly accelerated motion :
(i) v = u + at (ii) S = ut + l/2at2, where symbols have their usual meaning.
Answer:
Suppose, the initial velocity of a body is u and it is moving with uniform acceleration a for time t. Let the final velocity be v and the distance covered be S. Then, we have .

Question 14:
Neem and turmeric powders are often used in grain storage.
(i) What are they called?
(ii) What is the purpose of using neem and turmeric?
(iii) What are herbicides?
Answer:
(i) Bio-pesticide.
(ii) Neem and turmeric keep away insects, rodents, fungi, mites, bacteria etc. from the stored foods.
(iii) Herbicides are chemicals used for removal of weeds from cultivated fields during the early
stages of crop growth.
Question 15:
Shruti went to a hill station with her parents and her elder brother Rushil. One day they were walking through a valley where Shruti and her brother started playing. Shruti was surprised to distinctly hear the same sound twice. She discussed it with her brother. He told her that this phenomenon is called ’echo’.
(a) Define echo. What should be the minimum distance from the source for a distinct echo to be heard
(b) Write the values shown by Rushil?
Answer:
(a) Echo: It is the phenomenon of reflection of sound of a source from an obstacle. Minimum distance – 17.2 m
(b) Values shown by Rushil are – ready to listen to scientific facts, good listener.
Question 16:
(a) Give reason for the following :
(i) Why does a desert cooler cool better on a hot dry day ?
(ii) Why do we prefer to wear cotton clothes during summer ?
(b) Write any three differences between evaporation and boiling.
Answer:
(i) The humidity, i.e., the amount of water vapour in air is less on a hot dry day. Lesser the humidity, more is the rate of evaporation. Since the rate of evaporation is more on a hot dry day, a desert cooler cools better.
(ii) We perspire more during summer. Cotton is a good absorber of water. It absorbs sweat and exposes it for easy evaporation. As a result body feels cool and comfortable. So, we should wear cotton clothes during summer.
(b)
Boiling
|
Evaporation
|
(i) Boiling is a bulk phenomenon,
|
(i) Evaporation is a surface phenomenon.
|
(ii) Particles from the bulk of the liquid change into vapour state.
|
(ii)
Particles from the surface gain enough energy to overcome the
jntermolecular forces of attraction present in the liquid and change
into the vapour state.
|
(iii) Boiling starts at boiling point of a substance.
|
(iii) Evaporation starts below the boiling point.
|
(i) Identify in the situations given below where the object is making a uniform circular motion:
(a) A car turning around a curve with uniform speed. A car going uphill.
(b) Motion given to a discus by an athlete before releasing it. Motion of discus when the athlete releases it.
Give reason for your answer.
(ii) A cyclist completes five rounds of a circular track of radius 21 m in 12 minutes. Calculate his speed.
(iii) An artificial satellite moves around the earth with a velocity of constant magnitude, still its motion is said to be an accelerated motion, why ?
Answer:

(iii) Satellite is moving in a circular orbit with constant magnitude of velocity but its direction of motion is changing continuously, therefore, its motion is accelerated as the velocity changes in direction.
Question 18:
(a) How layers are different from broilers ? Give at least three differences between the two.
(b) What is composite fish culture system ? State one merit and one demerit of such a system.
Answer:
(a)
Layers
|
Broilers
|
(i) Layers are egg-laying chickens, managed for the purpose of getti ng eggs.
(ii)
Layers start producing eggs at an age of 20 weeks. So, they are kept
for longer period depending upon laying period (approx. 500 days).
(iii) They require enough space and adequate lighting.
|
(i) Broilers are maintained for getting meat.
(ii) They are raised up to 6-7 weeks in poultry farms and then sent to market for meat purposes.
(iii) They require conditions to grow fast and low mortality,
|
Demerit: Many of these fishes breed only during monsoon.
Question 19:
(a) Name the international organization who approves names of elements.
(b) Give an example with explanation to show that the Law of conservation of mass applies to physical changes also.
Answer:
(a) IUPAC (International Union of Pure and Applied Chemistry).
(b) Law of conservation of mass states that “mass can neither be created nor destroyed in a – chemical reaction.”
This law applies to physical changes also.
e.g., During interconversion of water into three states of matter there is no change in the mass of water taken although there is change in the three states.

Question 20:
Give reasons:
(a) Animals of phylum platyhelminthes are called flatworms.
(b) Bryophytes are called amphibians of the plant kingdom.
(c) Fungi are called saprophytes.
(d) Bacteria and tapeworms are very different in their body design.
(e) Plants like Pinus and Deodar are called gymnosperms.
Answer:
(a) Because they show such body design which is dorsiventrally flattened.
(b) Because they can live both on land and in water but they require water for fertilization as their gametes are non-motile.
(c) Because they use decaying organic material as food.
(d) Because bacterial belongs to kingdom monera and these organisms do not have a defined nucleus or organelles whereas tapeworm belongs to phylum platyhelminthes whose body is triplastic, bilaterally symmetrical, complex with true internal body cavity called coelom.
(e) Because they bear naked seeds and are usually perennial, evergreen and woody which are the features of gymnosperms.
Question 21:
(a) State Archimedes’ principle.
(b) It is easier to swim in sea water than in river water. Why ?
(c) Give any two important applications of Archimedes’ principle.
(d) What do you mean by relative density of a substance? What is its unit ?
OR
(a) Define power. Give its unit.
(b) A moving body of mass 20 kg has 40 Joules of kinetic energy. Calculate its speed.
(c) A person carrying a load of 20 kg climbs 4 m in 10 seconds. Calculate the work done and his power, (g = 10 m s-2)
Answer:
(a) Archimedes’ principle : When an object fully or partially immersed in a fluid, it experiences an upward thrust which is equal to the weight of fluid displaced by it.
(b) Because of higher density.
(c) 1. To check the purity of sample of milk.
2. To find the density of liquid.
(d) Relative density : It is the ratio of density of substance to the density of water. Units – It has no units
OR
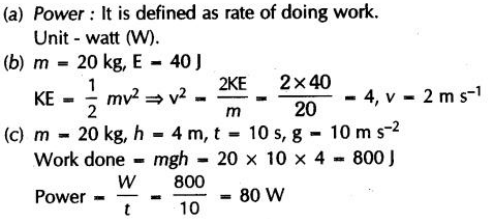
SECTION – B
Question 22:
(a) Mention the type of thermometer that should be used to determine the melting point of ice
in laboratory.
(b) While doing an experiment to determine the melting point of ice, state the role of glass stirrer.
Answer:
(a) Laboratory thermometer with minimum range of -10°C.
(b) Glass stirrer is used to mix the ice and ice cold water thoroughly to get a uniform temperature.
Question 23:
A substance ‘X’ was taken and was added to distilled water. On stirring thoroughly the aqueous solution was left to stand for sometime. It was observed that its particles began to settle at the bottom. By this information what do you infer about the solution? What would happen if a beam of light is passed through this solution ? Answer:
(i) The given solution is a suspension.
(ii) Light does not pass through and no Tyndall effect is observed.
Question 24:
List two precautions to be taken while you determine the mass percentage of water imbibed by raisins.
Answer:
(i) Only clean raisins should be used for the experiment.
(ii) Petri dish should be left undisturbed.
Question 25:
In a spring balance the space between 0 to 25 g wt is divided into 5 equal parts. Find its least count. What is the weight of the object in the diagram given below ?
Answer:

Question 26:
An object of volume 25mL suffers a loss in weight of 15 gwt when immersed fully in a solution. Find the density of the solution.
Answer:
Question 27:
(a) Why the body of bony fish is streamlined 1
(b) List two habitats of bony fish.
Answer:
(a) To reduce friction while swimming. It means that it offer least resistance to motion while swimming.
(b)
(i) Ponds
(ii) Lakes
(iii) Rivers
It’s habitat includes shallow water, having less salt concentration (known as fresh water) and higher levels of dissolved oxygen.
We hope the Solved CBSE Sample Papers for Class 9 Science Set 6, help you. If you have any query regarding CBSE Sample Papers for Class 9 Science Solved Set 6, drop a comment below and we will get back to you at the earliest
Subscribe to:
Comments (Atom)
AdSense
Download CLASS 10th CBSE Sample papers 2019
CBSE Sample Papers for Class 10 Science 2019 Solved Science Sample Question Paper 2019 Set 1 Solved Science Sample Question Paper 2019 ...
-
Q1.Describe three demands of Sri Lankan Tamils. How di d they struggle for their independence? Ans:A. Recognition of Tamil a...
-
Important Notes of LIGHT-REFLECTION & REFRACTION Light is a form of energy, which enable us to see the object. In this chapte...


